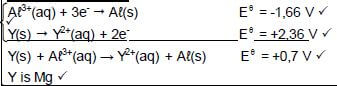Adele
GRADE 12 MATHEMATICS PAPER 1 QUESTIONS - NSC PAST PAPERS AND MEMOS SEPTEMBER 2017
GRADE 12 MATHEMATICS
PAPER 1
NSC PAST PAPERS AND MEMOS
SEPTEMBER 2017
INSTRUCTIONS AND INFORMATION
Read the following instructions carefully before answering the questions.
- This question paper consists of ELEVEN questions. Answer ALL the questions.
- Clearly show ALL calculations, diagrams, graphs, et cetera that you have used in determining your answer.
- You may use an approved scientific calculator (non-programmable and non graphical), unless stated otherwise.
- Answers only will not necessarily be awarded full marks.
- If necessary, round off answers to TWO decimal places, unless stated otherwise.
- Diagrams are NOT necessarily drawn to scale.
- Number the answers correctly according to the numbering system used in this question paper.
- Write neatly and legibly.
- An information sheet with formulae is included at the end of the question paper.
QUESTIONS
QUESTION 1
Solve for x:
1.1 2x(x+ 1) − 7(x + 1) = 0 (3)
1.2 x2 − 5x − 1 = 0 , correct to two decimal places. (3)
1.3 4x2 + 1 ≥ 5x (4)
1.4 54x+3. 100−2x+1 = 50 000 (4)
1.5 Solve for x and y simultaneously.
x = 2y and x2 + 2x − y − y2 = 36 (5)
1.6 Show that the roots of x2 − kx + k − 1 = 0 are real and rational for all real values of k. (4)
[23]
QUESTION 2
2.1 Given the following linear series: 3 + 7 + 11 + … … … + 483
2.1.1 How many terms does the above series have? (3)
2.1.2 Write the above series in sigma notation. (2)
2.2 2t − 4 ; t − 3 ; 8 − 2t are the 10th , 11th and 12th terms of an arithmetic sequence.
2.2.1 Determine the value of t . (3)
2.2.2 Calculate the value of the first term. (3)
2.3 The following information of a geometric pattern is given:
T1 + T2 = −1 and T3 + T4 = −4
Determine the numerical values of the first three terms if r > 0. (6)
[17]
QUESTION 3
Given the following list of numbers in a quadratic sequence:
41 ; 43 ; 47 ; 53 ; 61 ; 71 ; 83 ; 97 ; 113 ; 131
3.1 Determine the general term of the above sequence if it has a constant second difference. (5)
3.2 Calculate T41 and show that it is not a prime number. (3)
3.3 Determine the unit digit of the 49 999 998th term of the above sequence. (3)
[11]
QUESTION 4
4.1 Wayde invest R500 000 at 7,2% per annum compounded monthly.
4.1.1 Write down an expression for the value of his investment after n full years. (2)
4.1.2 Determine the value of his investment after 5 full years. (2)
4.1.3 If the investment exceeds R1 million rand after nfull years, calculate the value of n . (3)
4.2 Mr Jones wants to purchase a new car. The car cost R350 000 and he wants to pay R10 000 per month for three years. The interest rate on a financed loan, payable monthly, is 15% p.a. compounded monthly.
4.2.1 Calculate how much he will have to give as a deposit, to the auto dealer. (5)
4.2.2 Calculate his monthly payment if he pays no deposit and the car is financed over 5 years, at an interest rate of 18,5% p.a. compounded monthly. (4)
[16]
QUESTION 5
The sketch shows the graph of f(x) = x(x + 3) and g(x) = −½x + 2. 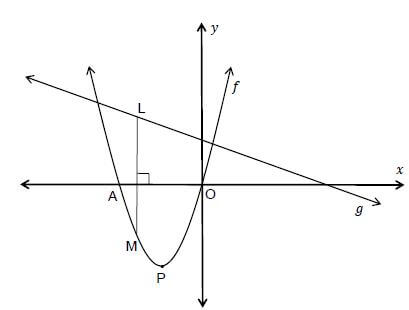
5.1 Determine the coordinates of A. (1)
5.2 Calculate the coordinates of P, the turning point of f . (3)
5.3 Determine the average gradient of f between x = −5 and x = −3 . (3)
5.4 Determine the value(s) of x for which f(x) > 0. (2)
5.5 Determine the coordinates of the turning point of ℎ if ℎ(x) = f(x − 2). (2)
5.6 L is a point on the straight line and M is a point on the parabola. LM is perpendicular to the x-axis. Show that the length LM can be written as:
LM = - [x + 7/4]2 + 81/16 (4)
[15]
QUESTION 6
Given: ℎ(x) = 2−x
6.1 Draw a neat sketch of ℎ . (3)
6.2 Determine the equation of q, the graph obtained by reflecting ℎ in the line y = 0 . (1)
6.3 Write down the equation of ℎ−1, the inverse of ℎ, in the form y = … (2)
6.4 Write down the range of ℎ . (1)
6.5 Sketch the graph of ℎ−1 on the same set of axes as ℎ . (2)
6.6 Determine the x-value(s) for which ℎ−1(x) ≥ −3 . (2)
[11]
QUESTION 7
A sketch of the hyperbola f(x ) = d - f
x - p , where p and d are constants, is given below.
The dotted lines are the asymptotes. The point A(5; 0) is given on the graph of f. 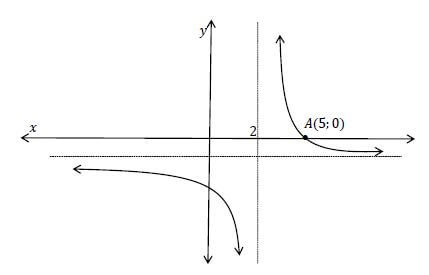
7.1 Determine the values of d and p . (2)
7.2 Show that the equation can be written as y = 3 - 1
x - 2 (2)
7.3 Write down the image of A if A is reflected about the axis of symmetry y = x − 3. (2)
[6]
QUESTION 8
8.1 Given: f(x) = −2x2 + p
Determine f′(x) from first principles. (5)
8.2 Determine: 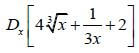 (4)
(4)
[9]
QUESTION 9
Given: f(x) = (x − 1)2(x + 3)
9.1 Determine the turning points of f. (5)
9.2 Draw a neat sketch of f showing all intercepts with the axes as well as the turning points. (4)
9.3 Determine the coordinates of the point where the concavity of f changes. (3)
9.4 Determine the value(s) of k, for which f(x) = k has three distinct roots. (2)
9.5 Determine the equation of the tangent to f that is parallel to the line y = −5x if x < 0 . (6)
[20]
QUESTION 10
A closed rectangular box, with a rectangle as base, has a length (2x) cm and width (x) cm. The total surface area (all 6 sides) is 243 cm2.
10.1 Show that the height, ℎ , is equal to [ 81 - 2x] cm
[ 2x 3 ]
10.2 Show that the volume of the box, in terms of x , is given by the formula:
V = 81x − 4/3x3 (2)
10.3 Calculate the value of x if the volume of the box is a maximum. (3)
[7]
QUESTION 11
11.1 You have to select a new password for your “Dropbox” account on your computer. The password must consist of 3 digits and 2 letters of the alphabet in that order. The digit 0(zero) is not allowed and no consonants are allowed. Any digit may be repeated but the vowels may not be repeated. How many different passwords are possible? (3)
11.2 Using the letters in the word FUNDAMENTALS , determine:
11.2.1 The number of unique 12 letter arrangements that can be formed (3)
11.2.2 The probability that a new arrangement will start and end with the letter N (3)
11.3 In a city where the ratio of male to female is 1 : 2, one person is selected to flip a fair coin for the kick-off of a soccer tournament.
11.3.1 Draw a tree diagram to show all possible outcomes of the coin toss. (3) 11.3.2 Determine the probability that it will be a woman who flips the coin. (1)
11.3.3 Determine the probability that it will be a man who flips a head. (2)
[15]
TOTAL: 150
PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
PHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
MEMORANDUM
QUESTION 1
1.1 B ✔✔ (2)
1.2 B ✔✔ (2)
1.3 A ✔✔ (2)
1.4 A ✔✔ (2)
1.5 C ✔✔ (2)
1.6 D ✔✔ (2)
1.7 C ✔✔ (2)
1.8 B ✔✔ (2)
1.9 C ✔✔ (2)
1.10 A ✔✔ (2)
[20]
QUESTION 2
2.1
2.1.1 B ✔ (1)
2.1.2 D OR/OF E ✔ (1)
2.1.3 F ✔ (1)
2.2
2.2.1 Butanal ✔ (1)
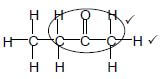
2.2.2 2,3,3-trimethyl✔but-1-ene ✔
Accept
2,3,3- trimethyl ✔-1- butene
Marking criteria:
|
 (3)
(3)
Marking criteria:
|
(2)
2.4
2.4.1 Esterification / Condensation ✔ (1)
2.4.2 Propan-1-ol ✔ ✔
If propanol (1 mark) (2)
2.4.3 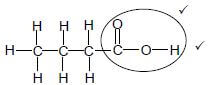
Marking criteria:
|
(2)
2.4.4 Propyl ✔ butanoate ✔(2)
[16]
QUESTION 3
3.1
- The temperature at which the vapour pressure equals atmospheric (external) pressure. ✔✔ (2 or 0) (2)
3.2
- Flammable / Catch fire easily. / Volatile ✔ (1)
3.3
3.3.1
- Use straight chain✔ primary alcohols ✔ (2)
3.3.2. OPTION 1
- Structure
Chain length / more C atoms in chain / molecular size / molecular mass / surface area increases from top to bottom / butan-1-ol to hexan-1-ol.✔ - Intermolecular forces
Intermolecular forces / Van der Waals forces / London forces / dispersion forces increases from top to bottom / butan-1-ol to hexan-1-ol. ✔ - Energy
Energy needed to overcome / break intermolecular forces increases from top to bottom / butan-1-ol to hexan-1-ol. ✔
OPTION 2
- Structure
Chain length / number of C atoms in the chain / molecular size / molecular mass/surface area decreases from bottom to top / hexan-1-ol to butan-1-ol. ✔ - Intermolecular forces
Intermolecular forces / Van der Waals forces/London forces / dispersion forces decreases from bottom to top/hexan-1-ol to butan-1-ol. ✔ - Energy
Energy needed to overcome / break intermolecular forces decreases from bottom to top / hexan-1-ol to butan-1-ol. ✔ (3)
3.4 Remains the same / Bly dieselfde ✔ (1)
3.5
3.5.1 Functional group / Type of homologous series ✔ (1)
3.5.2
- Type of intermolecular forces
Between molecules of aldehyde / hexanal are dipole-dipole forces. ✔ - Between molecules of alcohols / hexan-1ol are (in addition to dipole-dipole forces and London forces) hydrogen bonds. ✔
- Strength of intermolecular forces
Dipole-dipole forces are weaker than hydrogen bonds. ✔
OR
Hydrogen bonds are stronger than dipole-dipole forces. - Energy
More energy needed to overcome / break intermolecular forces in hexan-1-ol. ✔
OR
Less energy needed to overcome / break intermolecular forces in hexanal.✔ (4)
[14]
QUESTION 4
4.1
4.1.1 Substitution / hydrolysis ✔ (1)
4.1.2 H2O/water ✔
OR
Dilute sodium hydroxide /NaOH(aq)
OR
Dilute potassium hydroxide/KOH(aq)(1)
4.1.3 Tertiary ✔ (1)
4.1.4 Elimination / dehydrohalogenation / dehydrobromination ✔ (1) ✔✔
4.1.5 2-methylprop-1-ene / methylpropene / 2-methylpropene (2)
4.1.6 Halogenation / bromination ✔ (1)
4.1.7 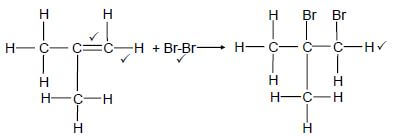 (4)
(4)
|
Notes:
|
4.2
4.2.1 Monomers ✔ (1)
4.2.2 Alkenes ✔ (1)
4.2.3 Addition (polymerisation) ✔ (1)
[14]
QUESTION 5/VRAAG 5
5.1 ANY TWO:
- Increase temperature of HCℓ. ✔
- Add a catalyst ✔
- Increase the concentration of HCℓ. ✔
- Increase the state of division of CuCO3 ✔
- Agitation / Stirring ✔ (2)
5.2 Accepted range : 42 s to 50 s ✔ (1)
5.3
5.3.
- average = - Δ m
Δ t
= - (169,76 - 170,000)✔
(20 - 0) ✔
= 0,012(g.s-1)✔
If answer is negative (minus 1 mark) (3)
5.3.2 Pure sample:
m(CO2)formed = 170,00 – 169,73 ✔
= 0,27 g
Impure sample:
m(CO2)formed = 170,00 - 169,78 ✔
= 0,22 g
%Purity = 0,22 × 100 ✔
0,27
= 81,48% ✔ (4)
5.3.3 POSITIVE MARKING FROM QUESTION 5.3.2.
- n(CO2) formed = m
M
= 0,27
44
= 6,13 × 10-3 mol
n(CO2) formed = V
VM
6,13 × 10-3 = V
22,4
V = 0,137 dm3 (3)
5.4 POSITIVE MARKING FROM QUESTION 5.2. 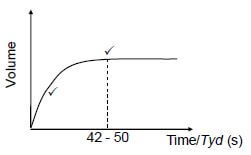
| Marking criteria for sketch graph: | |
Graph drawn from origin with decreasing gradient. | ✔ |
Constant volume after (42 -50) s.or graph stops at (42 -50) s | ✔ |
If no labels on axes: minus 1. |
(2)
[15]
QUESTION 6
6.1 Amount / number of moles / volume of (gas) reactants equals amount/number of moles/volume of (gas) products. ✔
OR
A change in pressure will change the concentration of the reactants and products equally. (1)
6.2
CALCULATIONS USING NUMBER OF MOLES
|
OPTION 1
KC = [HI]2
[H2][I2]
∴55,3 = [HI]2
(0,014)(0,0085)
∴[HI] = 0,08112 mol.dm-3
| No Kc expression, correct substitution Max. 8/9 | ||||
| Wrong Kc expression Max.6/9 | ||||
H2 | I2 | HI | ||
Initial mass (g) | (0,09812)(254) ✔ | |||
Initial quantity (mol) | 0,1091 | 0,09812 | 0 | |
Change (mol) | 0,08112 | 0,08112 ✔ | 0,1622 ✔ | using ratio |
Quantity at equilibrium (mol)/ | 0,028 | 0,017 | 0,1622 | |
Equilibrium concentration (mol∙dm-3) | 0,014 | 0,0085 | 0,08112 | × 2 |
| Divide by 2 ✔ | ||||
OR
KC = [HI]2
[H2][I2]
∴55,3 = X2
(0,014)(0,0085)
∴X = 0,08112 mol.dm-3
| No Kc expression, correct substitution Max. 8/9 | ||||
| Wrong Kc expression Max.6/9 | ||||
H2 | I2 | HI | ||
Initial mass (g) |
| |||
Initial quantity (mol) | x + 0,028 | x + 0,017 | 0 | |
Change (mol) | x | x | 2x | using ratio |
Quantity at equilibrium (mol)/ | 0,028 | 0,017 | 2x | |
Equilibrium concentration (mol∙dm-3) | 0,014 | 0,0085 | x | × 2 |
| Divide by 2 ✔ | ||||
Initial quantity I2(mol) (mol) = 0,08112 + 0,017
= 0,09812 mol
m(I2) = nM
= (0,09812)(254) ✔
= 24,92 g ✔
OPTION 2
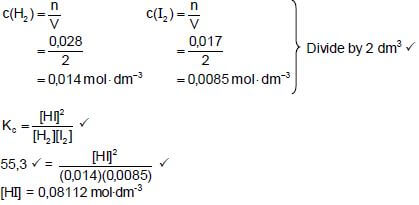
n(HI at equilibrium) = (0,08112)(2) = 0,1622 mol
n(HI formed) = n(HI at equilibrium) = 0,1622 mol
n(I2 reacted) = ½n(HI formed) = 0,08112 mol
n(I2 initial) = n(I2 reacted) + n(I2 equilibrium)
= 0,08112 + 0,017
= 0,09812 mol
m(I2 initial) = nM
= (0,09812)(254)
= 24,92 (g)✔
CALCULATIONS USING CONCENTRATION
|
OPTION 3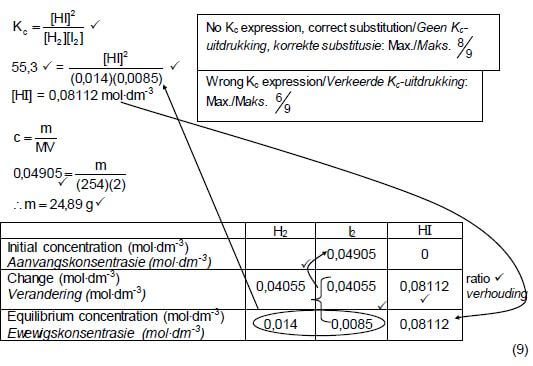
6.3 (Chemical/dynamic) equilibrium
OR
The rate of the forward reaction equals the rate of the reverse reaction. ✔ (1)
6.4
- Addition of a catalyst.
- Increase in pressure. (2)
6.5.1 Endothermic
- The rate of the forward reaction decreases more. / The rate of the reverse reaction decreases less.
- A decrease in temperature favours the exothermic reaction. (3)
6.5.2 Decreases (1)
6.6 Reactants / H2 / I2 removed
[18]
QUESTION 7
7.1 A substance that ionises incompletely/to a small extent.(2)
7.2
- Oxalic acid
- Higher Ka value
OR - Carbonic acid has a lower Ka value .(2)
7.3
- H2O
- (COO)2−2 (2)
7.4  (4)
(4)
7.5
7.5.1 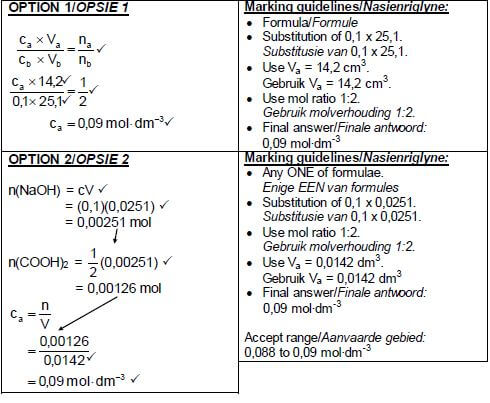 (5)
(5)
7.5.2
- C / phenolphthalein
- Titration of weak acid and strong base.
OR - The endpoint will be at pH > 7 which is in the range of the indicator. (2)
[17]
QUESTION 8
8.1
8.1.1 Salt bridge (1)
8.1.2 Voltaic / Galvanic cell (1)
8.2
8.2.1 Decreases(1)
8.2.2 Increases (1)
8.3
8.3.1 Y(s) → Y2+(aq) + 2e- Ignore phases
OR
Mg(s) → Mg2+(aq) + 2e -
Notes 0) Y2+(aq) + 2e- ⇌ Y(s) (0/2) Y(s) ← Y2+(aq) + 2e- (0/2) |
(2)
8.3.2 Y(s) |Y2+(aq) ||Aℓ3+(aq) | Aℓ(s) OR/OF Mg(s) |Mg2+(aq) ||Aℓ3+(aq) | Aℓ(s
OR
Y(s) | Y2+ (1 mol∙dm-3) || Aℓ3+(1 mol∙dm-3) | Aℓ(s)
Accept
Y | Y2+ || Aℓ3+ | Aℓ (3)
8.4 (5)
OPTION 1 0,7 = -1, 66 - Eθoxidation Eθoxidation = -2,36 (V) Y is Mg | Notes
|
OPTION 2
| |
[14]
QUESTION 9
9.1 Bauxite (1)
9.2 Oxidation (1)
9.3 Reduce melting point .
OR
To lower the temperature / energy needed to melt the Aℓ2O3. (1)
ACCEPT
|
9.4 Aℓ3+(aq) + 3e- → Aℓ(s)
Ignore phases
Notes |
(2)
9.5
- C + O2 → CO2 Bal
OR - 2Aℓ2O3 + 3C → 4Aℓ + 3CO2 Bal
Notes
|
(3)
[8]
QUESTION 10/VRAAG 10
10.1
10.1.1 Ostwald (process) (1)
10.1.2 Catalyst/Speeds up the rate of the reaction (1)
10.1.3 Nitrogen dioxide (1)
10.1.4 3NO2 + H2O ⇌ 2HNO3(aq) + NO Bal. (2)
Notes:
|
10.1.5
- Decrease pressure / Increase volume
- Decrease temperature \ (2)
10.2
10.2.1 (Ratio of the) nitrogen, phosphorous and potassium in the fertiliser. (1)
10.2.2 (6)
Marking criteria:
| ||
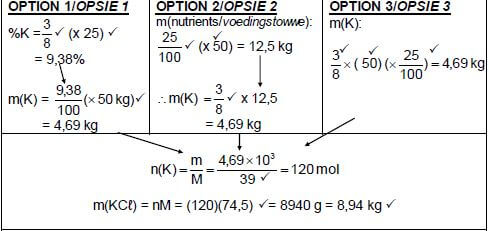 | ||
OPTION 4 m(K) = 9,38 x 50 = 4,69 kg m(100% KCℓ) = 4,69 x 100 |
[14]
TOTAL: 150
PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
PHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- Write your examination number and centre number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Write down the question number (1.1–1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE:
1.11 ![]()
1.1 Which ONE of the following is the product formed in the Haber process?
- Nitrogen
- Ammonia
- Nitric acid
- Sulphuric acid (2)
1.2 A carbonyl group is the functional group of …
- alcohols.
- ketones.
- haloalkanes.
- carboxylic acids. (2)
1.3 Consider the structure of an organic compound below. 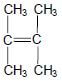
The IUPAC name of this compound is …
- 2,3-dimethylbut-2-ene.
- 2,2-dimethylbut-2-ene.
- 1,1,2-trimethylprop-1-ene.
- 1,1,2,2-tetramethylethene. (2)
1.4 Consider the reaction represented below.
CH3CH2CH2CH2CH3 → CH3CHCH2 + X
Which ONE of the following CORRECTLY gives the type of reaction that takes place and the IUPAC name of product X?
Type of reaction | Product X | |
A | Elimination | Ethane |
B | Elimination | Ethene |
C | Addition | Ethane |
D | Addition | Ethene |
(2)
1.5 Consider the following balanced equation of a chemical reaction:
2NaCℓ + 2H2O → Cℓ2 + H2 + 2NaOH
Which ONE of the following statements about the reaction is correct?
The reaction takes place in a/an …
- galvanic cell and absorbs energy.
- galvanic cell and releases energy.
- electrolytic cell and absorbs energy.
- electrolytic cell and releases energy. (2)
1.6 The following equation represents the reaction taking place in an electrochemical cell:
Ni(s) + Pb2+(aq) → Ni2+(aq) + Pb(s)
The flow of electrons through the external circuit of this cell is from …
- Pb at the anode to Ni at the cathode.
- Pb at the cathode to Ni at the anode.
- Ni at the cathode to Pb at the anode.
- Ni at the anode to Pb at the cathode. (2)
1.7 A solution has a pH = 1. This solution …
- contains no OH─ ions.
- neutralises a hydrochloric acid solution of pH = 1.
- contains a higher concentration of H3O+ ions than OH─ ions.
- contains a higher concentration of OH─ ions than H3O+ ions. (2)
1.8 A potential energy diagram can be used to show the activation energy (EA) and the heat of reaction (ΔH) of a reaction.
Which ONE of the following combinations of values of EA and ΔH CANNOT be obtained for any reaction?
EA (kJ·mol-1) | ∆H (kJ·mol-1) | |
A | 50 | -100 |
B | 50 | +100 |
C | 100 | +50 |
D | 100 | -50 |
(2)
1.9 Initially, 2 mol CO(g) and 2 mol H2(g) are sealed in a container. The reaction reaches equilibrium according to the following balanced equation:
CO(g) + 2H2(g) ⇌ CH3OH(g)
At equilibrium the amount of CH3OH(g) in the mixture will be …
- 1 mol.
- 2 mol.
- less than 1 mol.
- greater than 1 mol. (2)
1.10 The graph below represents the change in concentration of a reactant against time for a chemical reaction. 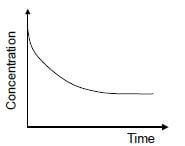
In which ONE of the following graphs does the dotted line show the effect of a catalyst on this reactant? 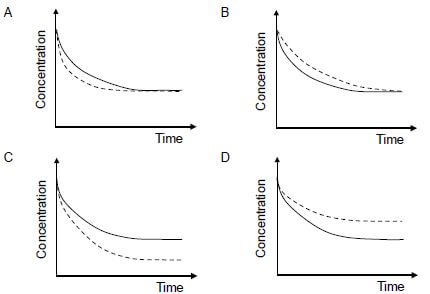 (2)
(2)
[20]
QUESTION 2 (Start on a new page.)
The letters A to F in the table below represent six organic compounds. 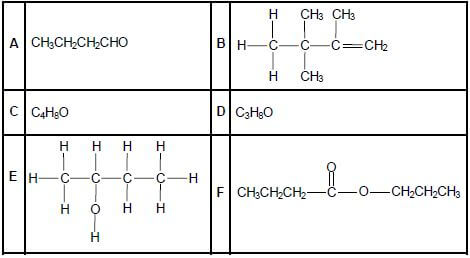
2.1 Write down the letter that represents EACH of the following:
2.1.1 A hydrocarbon (1)
2.1.2 An alcohol (1)
2.1.3 An ester (1)
2.2 Write down the IUPAC name of:
2.2.1 Compound A (1)
2.2.2 Compound B (3)
2.3 Compound C is a functional isomer of compound A. Write down the structural formula of compound C. (2)
2.4 Compound D is used as one of the reactants to prepare compound F. Write down the:
2.4.1 Type of reaction which takes place to prepare compound F (1)
2.4.2 IUPAC name of compound D (2)
2.4.3 Structural formula of the other organic reactant used (2)
2.4.4 IUPAC name of compound F (2)
[16]
QUESTION 3 (Start on a new page.)
Learners investigate factors which influence the boiling points of alcohols.
They use equal volumes of each of the alcohols and heat them separately in a water bath. The temperature at which each boils is measured. The results obtained are shown in the table below.
ALCOHOLS | BOILING POINTS OF ALCOHOLS |
Butan-1-ol | 117,7 |
Pentan-1-ol | 138,5 |
Hexan-1-ol | 157,0 |
3.1 Define the term boiling point. (2)
3.2 What property of alcohols requires them to be heated in a water bath? (1)
3.3 The boiling points of the alcohols are compared with each other.
3.3.1 What structural requirements must the alcohols meet to make it a fair comparison? (2)
3.3.2 Fully explain the trend in the boiling points. (3)
3.4 How will the boiling point of hexan-1-ol be affected if the volume of hexan-1-ol used is doubled? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
3.5 In another investigation the learners compare the boiling points of hexan-1-ol and hexanal.
3.5.1 Write down the independent variable for this comparison. (1)
3.5.2 They find that the boiling point of hexan-1-ol is higher than that of hexanal. Fully explain this observation. (4)
[14]
QUESTION 4 (Start on a new page.)
4.1 Consider the reactions represented in the flow diagram below.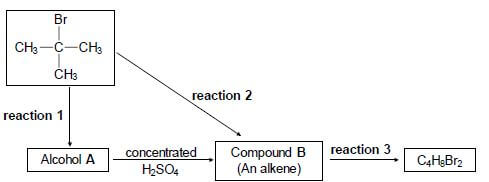
Write down the:
4.1.1 Type of reaction represented by reaction 1 (1)
4.1.2 NAME or FORMULA of the inorganic reactant needed for reaction 1 (1)
4.1.3 Type of alcohol (PRIMARY, SECONDARY or TERTIARY) of which alcohol A is an example (1)
4.1.4 Type of reaction represented by reaction 2 (1)
4.1.5 IUPAC name of compound B (2)
4.1.6 Type of addition reaction represented by reaction 3 (1)
4.1.7 Balanced equation for reaction 3 using structural formulae (4)
4.2 A wide range of synthetic polymers are produced by combining large numbers of similar small organic molecules bonded to each other in a repeating pattern.
Polymer C below is an example of such a polymer. 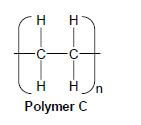
Write down:
4.2.1 ONE word for the underlined phrase (1)
4.2.2 The homologous series to which the 'small organic molecules' used to produce polymer C belong (1)
4.2.3 The type of polymerisation which takes place to produce polymer C (1)
[14]
QUESTION 5 (Start on a new page.)
The reaction of copper(II) carbonate with excess dilute hydrochloric acid is used to investigate the rate of reaction. The balanced equation for the reaction is:
CuCO3(s) + 2HCℓ(aq) → CuCℓ2(aq) + H2O(ℓ) + CO2(g)
The apparatus used is illustrated below. 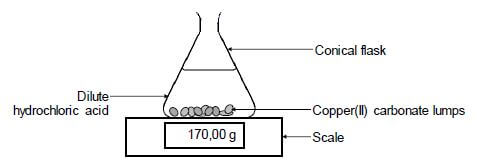
5.1 State TWO ways in which the rate of the reaction above can be increased. (2)
During the investigation, samples of both PURE and IMPURE copper(II) carbonate of EQUAL mass are used. The graphs below are obtained from the results. 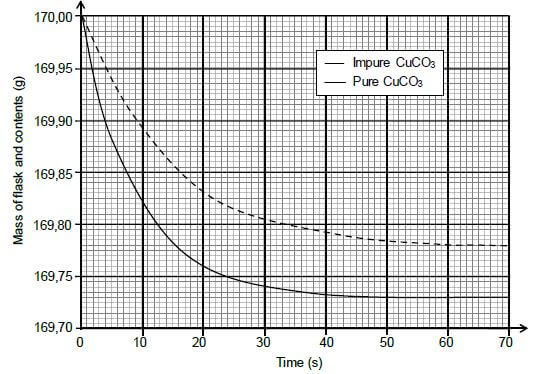
5.2 Write down the reaction time for the reaction of the pure CuCO3 with HCℓ. (1)
5.3 Assume that all the gas formed during the two reactions escape from the flask and that the impurities do not react.
Calculate the:
5.3.1 Average rate of the reaction of the pure sample over the first 20 s (3)
5.3.2 Percentage purity of the impure sample (4)
5.3.3 Maximum volume of CO2(g) produced during the reaction of the pure sample of CuCO3 if the reaction takes place at STANDARD CONDITIONS (3)
5.4 Sketch a graph of the volume of gas produced versus time for the reaction of the pure CuCO3. Indicate the reaction time on the x-axis. (2)
[15]
QUESTION 6 (Start on a new page.)
Hydrogen and iodine are sealed in a 2 dm3 container. The reaction is allowed to reach equilibrium at 700 K according to the following balanced equation:
H2(g) + I2(g) ⇌ 2HI(g)
6.1 Give a reason why changes in pressure will have no effect on the equilibrium position. (1)
6.2 At equilibrium, 0,028 mol H2(g) and 0,017 mol I2(g) are present in the container.
Calculate the initial mass of I2(g), in grams, that was sealed in the container, if Kc for the reaction is 55,3 at 700 K. (9)
The reaction rate versus time graph below represents different changes made to the equilibrium mixture. 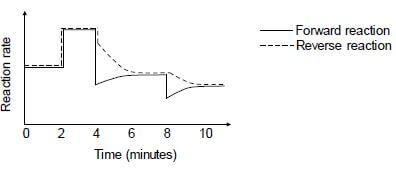
6.3 What do the parallel lines in the first two minutes indicate? (1)
6.4 State TWO possible changes that could be made to the reaction conditions at t = 2 minutes. (2)
6.5 The temperature of the equilibrium mixture was changed at t = 4 minutes.
6.5.1 Is the forward reaction EXOTHERMIC or ENDOTHERMIC? Fully explain the answer. (3)
6.5.2 How will this change influence the Kc value? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
6.6 What change was made to the equilibrium mixture at t = 8 minutes? (1)
[18]
QUESTION 7 (Start on a new page.)
The Ka values for two weak acids, oxalic acid and carbonic acid, are as follows:
NAME | FORMULA | Ka |
Oxalic acid | (COOH)2 | 5,6 x 10-2 |
Carbonic acid | H2CO3 | 4,3 x 10-7 |
7.1 Define the term weak acid. (2)
7.2 Which acid, OXALIC ACID or CARBONIC ACID, is stronger? Give a reason for the answer. (2)
7.3 Oxalic acid ionises in water according to the following balanced equation:
(COOH)2(s) + 2H2O(ℓ) ⇌ (COO)2−2 (aq) + 2H3O+(aq)
Write down the FORMULAE of the TWO bases in this equation. (2)
7.4 Learners prepare 2 dm3 of a sodium hydroxide solution of concentration 0,1 mol∙dm-3. Calculate the pH of the solution. (4)
7.5 During a titration of the sodium hydroxide solution in QUESTION 7.4 with dilute oxalic acid, the learners find that 25,1 cm3 of the NaOH(aq) neutralises exactly 14,2 cm3 of the (COOH)2(aq).
The balanced equation for the reaction is as follows:
2NaOH(aq) + (COOH)2(aq) → (COO)2Na2(aq) + 2H2O(ℓ)
7.5.1 Calculate the concentration of the oxalic acid solution. (5) The following indicators are available for the titration:
INDICATOR | pH RANGE |
A | 3,1–4,4 |
B | 6,0–7,6 |
C | 8,3–10,0 |
7.5.2 Which ONE of the indicators above is most suitable for this titration? Give a reason for the answer. (2)
[17]
QUESTION 8 (Start on a new page.)
In the electrochemical cell shown below an aluminium electrode and another metal electrode, Y, are used. 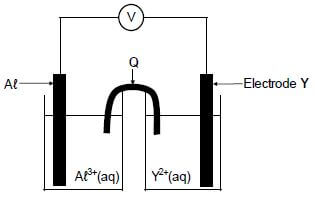
8.1 Write down the:
8.1.1 Name of component Q (1)
8.1.2 Type of electrochemical cell represented above (1)
It is found that the mass of the aluminium electrode increases whilst the cell is functioning.
8.2 How will EACH of the following change while the cell is functioning? Choose from INCREASES, DECREASES or REMAINS THE SAME.
8.2.1 The concentration of Aℓ3+(aq) (1)
8.2.2 The concentration of Y2+(aq) (1)
8.3 Write down the:
8.3.1 Half-reaction that takes place at electrode Y (2)
8.3.2 Cell notation of the cell (3)
8.4 The initial emf of this cell measured under standard conditions is 0,7 V. Identify metal Y by means of a calculation. (5)
[14]
QUESTION 9 (Start on a new page.)
The simplified diagram below shows an electrolytic cell used in the industrial extraction of aluminium (Aℓ) from aluminium oxide at temperatures as high as 1 000 ºC.
Electrode X is a carbon rod. 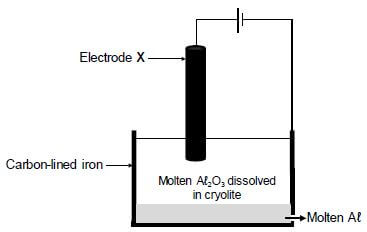
The cell reaction that takes place is as follows:
2Aℓ2O3(ℓ) → 4Aℓ(ℓ) + 3O2(g)
9.1 Write down the name of the ore used as source of aluminium oxide. (1)
9.2 Which half-reaction (OXIDATION or REDUCTION) takes place at electrode X? (1)
9.3 What is the function of the cryolite? (1) 9.4 Write down the reduction half-reaction. (2)
9.5 Write down a balanced equation that shows why the carbon rod, X, must be replaced regularly. (3)
[8]
QUESTION 10 (Start on a new page.)
10.1 The reactions represented below take place during one of the industrial processes used in the fertiliser industry.
PT
- : 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g) ∆H < 0
- NO(g) + O2(g) ⇌ X
- : NO2 + H2O(ℓ) ⇌ HNO3(aq) + _____
Write down:
10.1.1 The name of this industrial process (1)
10.1.2 The function of Pt in reaction I (1)
10.1.3 The NAME of product X (1)
10.1.4 A balanced equation for reaction III (2)
10.1.5 TWO ways in which the yield of the NO(g) obtained in reaction I can be increased without changing the amount of reactants and products (2)
10.2 NPK fertilisers contain NH4NO3, (NH4)3PO4 and KCℓ in varying proportions.
10.2.1 What does NPK mean? (1)
10.2.2 Consider the fertiliser illustrated below. 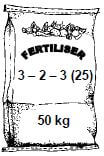
Calculate the mass, in kg, of KCℓ needed to produce this fertiliser. (6)
[14]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 x 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 x 10-19 C |
Avogadro's constant | NA | 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE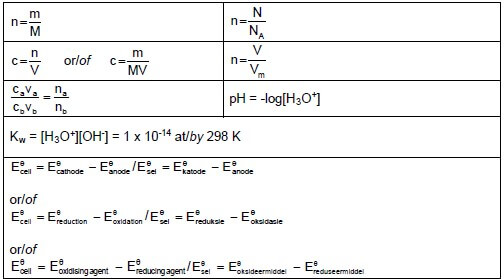
TABLE 3: THE PERIODIC TABLE OF ELEMENTS 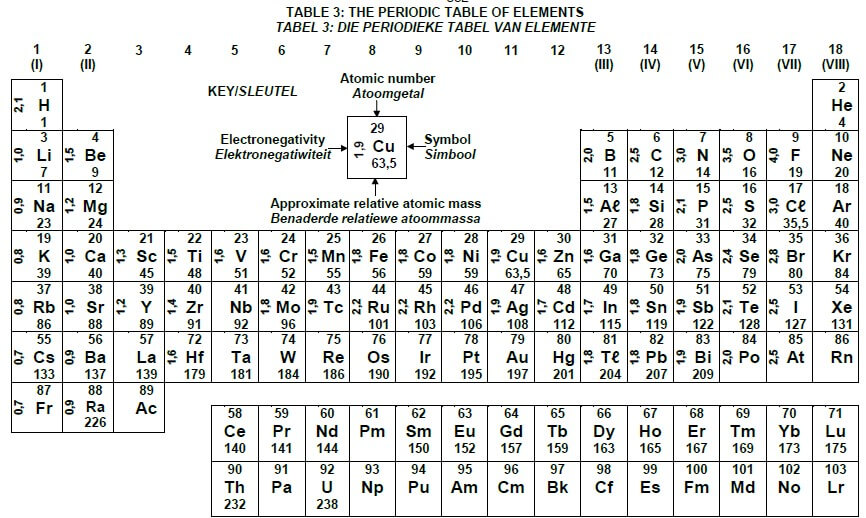
TABLE 4A: STANDARD REDUCTION POTENTIALS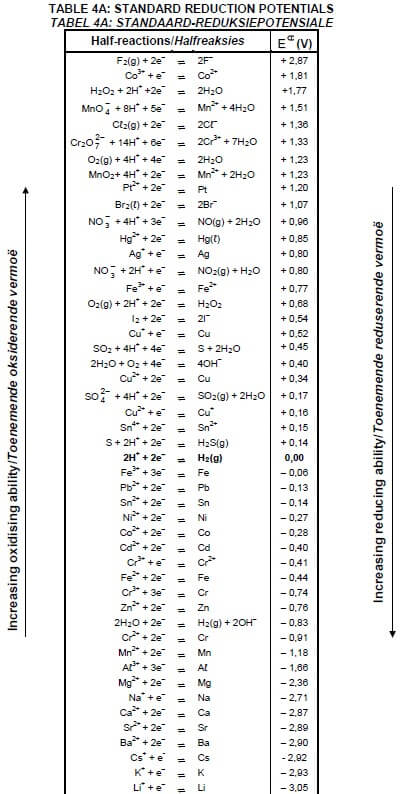
TABLE 4B: STANDARD REDUCTION POTENTIALS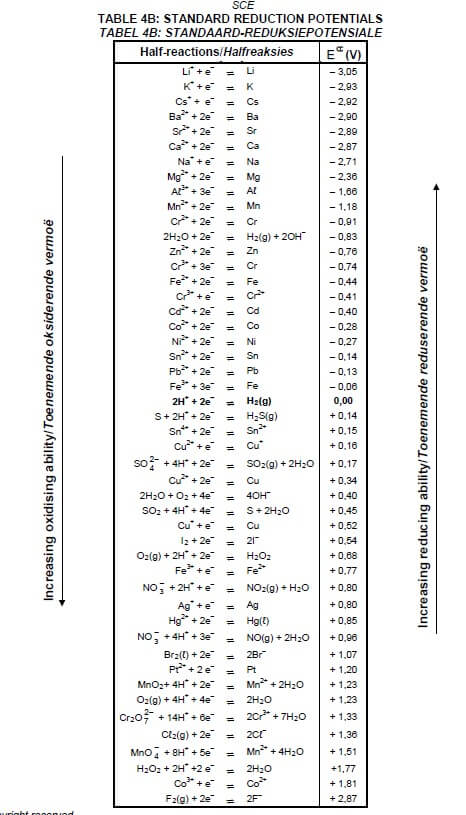
PHYSICAL SCIENCES: PHYSICS PAPER 1 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
PHYSICAL SCIENCES: PHYSICS
PAPER 1
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of 10 questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Write down the question number (1.1–1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE:
1.11 ![]()
1.1 According to Newton's Second Law of Motion, the acceleration of an object is …
- independent of its mass.
- always equal to its mass.
- directly proportional to its mass.
- inversely proportional to its mass. (2)
1.2 The diagram below shows three blocks, P, Q and R, suspended from a ceiling. The blocks are identical, stationary and have the same mass but are at different heights above the ground.
The connecting strings are massless and inextensible. The tensions in the strings attached to blocks P, Q and R are TP, TQ and TR respectively. 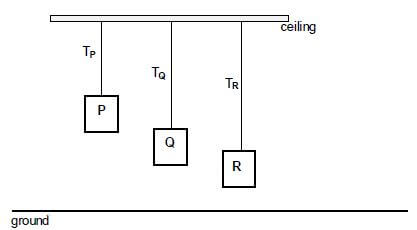
Which ONE of the following statements about the tensions is CORRECT?
- TP > TQ > TR
- TP < TQ < TR
- TP = TQ = TR
- TP > TQ and TQ < TR (2)
1.3 A ball is projected vertically upwards from the ground. It returns to the ground, makes an elastic collision with the ground and then bounces to a maximum height. Ignore air resistance.
Which ONE of the following velocity-time graphs CORRECTLY describes the motion of the ball? 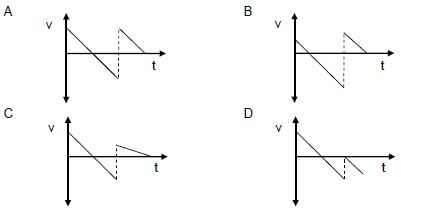 (2)
(2)
1.4 When the velocity of a moving object is doubled, the …
- net work done by the object is doubled.
- kinetic energy of the object is doubled.
- potential energy of the object is doubled.
- linear momentum of the object is doubled. (2)
1.5 The net work required to stop a moving object is equal to the …
- inertia of the object.
- change in kinetic energy of the object.
- change in momentum of the object.
- change in impulse of the object. (2)
1.6 A stationary observer is listening to the sound coming from a sound source. The listener hears a sound of a lower pitch when compared to that produced by the source.
What can you conclude about the source from this observation?
- The source is at rest.
- The source is moving towards the listener.
- The source is moving away from the listener.
- There is an obstacle between the source and the listener. (2)
1.7 Two charged particles are placed a distance, r, apart. The electrostatic force exerted by one charged particle on the other is FE.
Which ONE of the graphs below CORRECTLY represents the relationship between the electrostatic force, FE, and the square of the distance, r2, between the two charges? 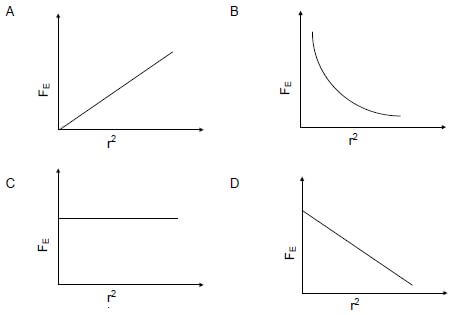 (2)
(2)
1.8 In the circuit diagram below, the resistance of resistor R1 is TWICE the resistance of resistor R2.
The two resistors are connected in series and identical high-resistance voltmeters are connected across each resistor.
The readings on the voltmeters are V1 and V2 respectively. 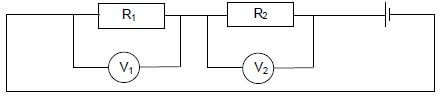
Which ONE of the following statements concerning the voltmeter readings is CORRECT?
- V1 = 2V2
- V1 = ½V2
- V1 = ¼V2
- 2V1 = V2 (2)
1.9 In a DC generator the current to the external circuit is delivered through the …
- coils.
- battery.
- slip rings.
- split rings (commutators). (2)
1.10 In an experiment on the photoelectric effect, the frequency of the incident light is high enough to cause the removal of electrons from the surface of the metal.
The number of electrons ejected from the metal surface is proportional to the …
- kinetic energy of the electrons.
- number of incident photons.
- work function of the metal.
- frequency of the incident light. (2)
[20]
QUESTION 2 (Start on a new page.)
In the diagram below, a small object of mass 2 kg is sliding at a constant velocity of 1,5 m⋅s-1 down a rough plane inclined at 7º to the horizontal surface. 
At the bottom of the plane, the object continues sliding onto the rough horizontal surface and eventually comes to a stop.
The coefficient of kinetic friction between the object and the surface is the same for both the inclined surface and the horizontal surface.
2.1 Write down the magnitude of the net force acting on the object. (1)
2.2 Draw a labelled free-body diagram for the object while it is on the inclined plane. (3)
2.3 Calculate the:
2.3.1 Magnitude of the frictional force acting on the object while it is sliding down the inclined plane (3)
2.3.2 Coefficient of kinetic friction between the object and the surfaces (3)
2.3.3 Distance the object travels on the horizontal surface before it comes to a stop (5)
[15]
QUESTION 3 (Start on a new page.)
A hot-air balloon moves vertically downwards at a constant velocity of 1,2 m∙s-1. When it reaches a height of 22 m from the ground, a ball is dropped from the balloon.
Refer to the diagram below. 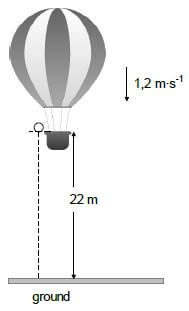
Assume that the dropping of the ball has no effect on the speed of the hot-air balloon. Ignore air friction for the motion of the ball.
3.1 Explain the term projectile motion. (2)
3.2 Is the hot-air balloon in free fall? Give a reason for the answer. (2)
3.3 Calculate the time it takes for the ball to hit the ground after it is dropped. (4)
When the ball lands on the ground, it is in contact with the ground for 0,3 s and then it bounces vertically upwards with a speed of 15 m∙s-1.
3.4 Calculate how high the balloon is from the ground when the ball reaches its maximum height after the first bounce. (6)
[14]
QUESTION 4 (Start on a new page.)
4.1 Define the term impulse in words. (2)
4.2 The diagram below shows a gun mounted on a mechanical support which is fixed to the ground. The gun is capable of firing bullets rapidly in a horizontal direction.
Each bullet travels at a speed of 700 m∙s-1 in an easterly direction when it leaves the gun.
(Take the initial velocity of a bullet, before being fired, as zero.) 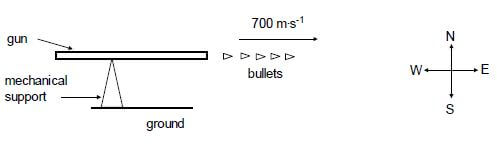
The gun fires 220 bullets per minute. The mass of each bullet is 0,03 kg. Calculate the:
4.2.1 Magnitude of the momentum of each bullet when it leaves the gun (3)
4.2.2 The net average force that each bullet exerts on the gun (5)
4.3 Without any further calculation, write down the net average horizontal force that the mechanical support exerts on the gun. (2)
[12]
QUESTION 5 (Start on a new page.)
A lift arrangement comprises an electric motor, a cage and its counterweight. The counterweight moves vertically downwards as the cage moves upwards. The cage and counterweight move at the same constant speed. Refer to the diagram below. 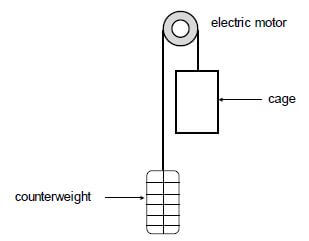
The cage, carrying passengers, moves vertically upwards at a constant speed, covering 55 m in 3 minutes. The counterweight has a mass of 950 kg. The total mass of the cage and passengers is 1 200 kg. The electric motor provides the power needed to operate the lift system. Ignore the effects of friction.
5.1 Define the term power in words. (2)
5.2 Calculate the work done by the:
5.2.1 Gravitational force on the cage (3)
5.2.2 Counterweight on the cage (2)
5.3 Calculate the average power required by the motor to operate the lift arrangement in 3 minutes. Assume that there are no energy losses due to heat and sound. (6)
[13]
QUESTION 6 (Start on a new page.)
6.1 A sound source is moving at constant velocity past a stationary observer. The frequency detected as the source approaches the observer is 2 600 Hz. The frequency detected as the source moves away from the observer is 1 750 Hz.
Take the speed of sound in air as 340 m∙s-1
6.1.1 Name the phenomenon that describes the apparent change in frequency detected by the observer. (1)
6.1.2 State ONE practical application of the phenomenon in QUESTION 6.1.1 in the field of medicine. (1)
6.1.3 Calculate the speed of the moving source. (6)
6.1.4 Will the observed frequency INCREASE, DECREASE or REMAIN THE SAME if the velocity of the source increased as it:
- Moves towards the observer (1)
- Moves away from the observer (1)
6.2 Spectral lines of star X at an observatory are observed to be red shifted.
6.2.1 Explain the term red shifted in terms of wavelength. (2)
6.2.2 Will the frequency of the light observed from the star INCREASE, DECREASE or REMAIN THE SAME? (1)
[13]
QUESTION 7 (Start on a new page.)
7.1 A metal sphere A, suspended from a wooden beam by means of a non-conducting string, has a charge of +6 µC.
7.1.1 Were electrons ADDED TO or REMOVED FROM the sphere to obtain this charge? Assume that the sphere was initially neutral. (1)
7.1.2 Calculate the number of electrons added to or removed from the sphere. (3)
7.2 Point charges Q1, Q2 and Q3 are arranged at the corners of a right-angled triangle, as shown in the diagram below. 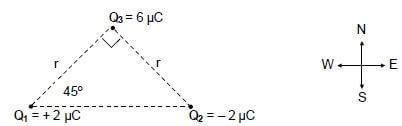
The charges on Q1 and Q2 are + 2 µC and – 2 µC respectively and the magnitude of the charge on Q3 is 6 µC.
The distance between Q1 and Q3 is r. The distance between Q2 and Q3 is also r.
The charge Q3 experiences a resultant electrostatic force of 0,12 N to the west.
7.2.1 Without calculation, identify the sign (positive or negative) on the charge Q3. (1)
7.2.2 Draw a vector diagram to show the electrostatic forces acting on Q3 due to charges Q1 and Q2 respectively. (2)
7.2.3 Write down an expression, in terms of r, for the horizontal component of the electrostatic force exerted on Q3 by Q1. (3)
7.2.4 Calculate the distance r. (4)
7.3 The magnitude of the electric field is 100 N·C-1 at a point which is 0,6 m away from a point charge Q.
7.3.1 Define the term electric field at a point in words. (2)
7.3.2 Calculate the distance from point charge Q at which the magnitude of the electric field is 50 N∙C-1. (5)
[21]
QUESTION 8 (Start on a new page).
8.1 In Circuit 1 below three identical light bulbs, P, Q and R, with the same resistance, are connected to a battery with emf ε and negligible internal resistance. 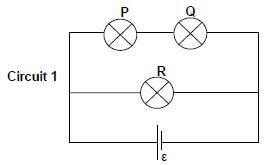
8.1.1 How does the brightness of bulb P compare with that of bulb Q? Give a reason for the answer. (2)
8.1.2 How does the brightness of bulb P compare with that of bulb R? Give a reason for the answer. (2)
A fourth, identical bulb T, with the same resistance as the other three, is connected to the circuit by means of an ordinary wire of negligible resistance, as shown in Circuit 2 below. 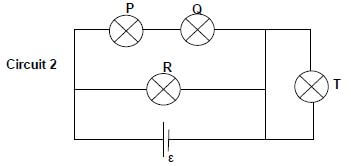
8.1.3 How does the brightness of bulb T compare with that of bulb R? Give a reason for the answer. (2)
8.2 A battery with an emf of 20 V and an internal resistance of 1 Ω is connected to three resistors, as shown in the circuit below. 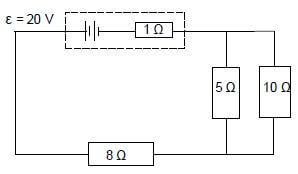
Calculate the:
8.2.1 Current in the 8 Ω resistor (6)
8.2.2 Potential difference across the 5 Ω resistor (4)
8.2.3 Total power supplied by the battery (3)
[19]
QUESTION 9 (Start on a new page.)
The diagram below shows a simplified version of an AC generator.
9.1 Name the component in this arrangement that makes it different from a DC generator. (1)
9.2 Sketch a graph of induced emf versus time for TWO complete rotations of the coil. (2)
A practical version of the generator above has a large number of turns of the coil and it produces an rms potential difference of 240 V.
9.3 State TWO ways in which the induced emf can be increased. (2)
9.4 Define the term root mean square (rms) value of an AC potential difference. (2)
9.5 The practical version of the generator above is connected across an appliance rated at 1 500 W. Calculate the rms current passing through the appliance. (3)
[10]
QUESTION 10 (Start on a new page.)
The graph below is obtained for an experiment on the photoelectric effect using different frequencies of light and a given metal plate. 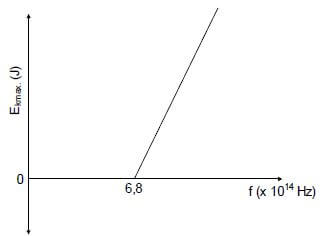
The threshold frequency for the metal is 6,8 x 1014 Hz.
10.1 Define the term threshold frequency. (2)
In the experiment, the brightness of the light incident on the metal surface is increased.
10.2 State how this change will influence the speed of the photoelectrons emitted.
Choose from INCREASES, DECREASES or REMAINS UNCHANGED. (1)
10.3 Show by means of a calculation whether the photoelectric effect will be OBSERVED or NOT OBSERVED, if monochromatic light with a wavelength of 6 x 10-7 m is used in this experiment. (5)
One of the radiations used in this experiment has a frequency of 7,8 x 1014 Hz.
10.4 Calculate the maximum speed of an ejected photoelectron. (5)
[13]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 1 (PHYSICS)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Acceleration due to gravity | g | 9,8 m•s-2 |
Universal gravitational constant | G | 6,67 × 10-11 N•m2•kg-2 |
Speed of light in a vacuum | c | 3,0 × 108 m•s-1 |
Planck's constant | h | 6,63 × 10-34 J•s |
Coulomb's constant | k | 9,0 × 109 N•m2•C-2 |
Charge on electron | e | -1,6 × 10-19 C |
Electron mass | me | 9,11 × 10-31 kg |
Mass of earth | M | 5,98 × 1024 kg |
Radius of earth | RE | 6,38 × 103 km |
TABLE 2: FORMULAE
MOTION
| vf = vi + aΔt | Δx = ViΔt + ½aΔt2 or Δy = ViΔt2 + ½aΔt2 |
Vf2 = Vi2 + 2aΔx or Vf2 = vi2 + 2aΔy | Δx = [Vi + Vf]Δt or Δy = [Vi + Vf]Δt |
FORCE
Fnet = ma | p= mv |
fsmax = µsN | fk = µkN |
FnetΔt = Δp | w =mg |
F = Gm1m2 | g = G M |
WORK, ENERGY AND POWER
W =FΔxcosθ | U= mgh or EP = mgh |
K = ½mv2 or Ek = ½mv2 | Wnet = ΔK or Wnet = ΔEk ΔK = Kf −Ki or ΔEk =Ekf − Eki |
Wnc= ΔK + ΔU or Wnc= ΔEk + ΔEp | P = W Δt |
Pav = Fv |
WAVES, SOUND AND LIGHT
v = f λ | T =1/f |
fl = v ± vl fs fl = v ± vl fb | E = hf or E = h c |
E = W0 + Ek where | |
ELECTROSTATICS
| F = kQ1Q2 r2 | E = KQ |
E = V | E = F |
V = W | n = Q |
ELECTRIC CIRCUITS
R = V | emf (ε) = I(R + r) |
RS = R1 + R2 + ....... | q = I Δt |
W = Vq | P= W |
ALTERNATING CURRENT
I rms = Imax | Paverage = VrmsIrms |
PHYSICAL SCIENCES: PHYSICS PAPER 1 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
PHYSICAL SCIENCES: PHYSICS
PAPER 1
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
GENERAL MARKING GUIDELINES
1. CALCULATIONS
1.1 Marks will be awarded for: correct formula, correct substitution, correct answer with unit.
1.2 No marks will be awarded if an incorrect or inappropriate formula is used, even though there may be relevant symbols and applicable substitutions.
When an error is made during substitution into a correct formula, a mark will be awarded for the correct formula and for the correct substitutions, but no further marks will be given.
1.3 If no formula is given, but all substitutions are correct, a candidate will forfeit one mark.
1.4 No penalisation if zero substitutions are omitted in calculations where correct formula/principle is given correctly.
1.5 Mathematical manipulations and change of subject of appropriate formulae carry no marks, but if a candidate starts off with the correct formula and then changes the subject of the formula incorrectly, marks will be awarded for the formula and the correct substitutions. The mark for the incorrect numerical answer is forfeited.
1.6 Marks are only awarded for a formula if a calculation has been attempted, i.e. substitutions have been made or a numerical answer given.
1.7 Marks can only be allocated for substitutions when values are substituted into formulae and not when listed before a calculation starts.
1.8 All calculations, when not specified in the question, must be done to a minimum of TWO decimal places.
1.9 If a final answer to a calculation is correct, full marks will not automatically be awarded. Markers will always ensure that the correct/appropriate formula is used and that workings, including substitutions, are correct.
1.10 Questions where a series of calculations have to be made (e.g. a circuit diagram question) do not necessarily always have to follow the same order. FULL MARKS will be awarded provided it is a valid solution to the problem. However, any calculation that will not bring the candidate closer to the answer than the original data, will not count any marks.
2. UNITS
2.1 Candidates will only be penalised once for the repeated use of an incorrect unit within a question.
2.2 Units are only required in the final answer to a calculation. Eenhede word slegs in die finale antwoord op 'n vraag verlang.
2.3 Marks are only awarded for an answer, and not for a unit per se. Candidates will therefore forfeit the mark allocated for the answer in each of the following situations:
- Correct answer + wrong unit
- Wrong answer + correct unit
- Correct answer + no unit
2.4 SI units must be used except in certain cases, e.g. V∙m-1instead of N∙C-1, and cm∙s-1or km∙h-1 instead of m∙s-1 where the question warrants this.
3. GENERAL
3.1 If one answer or calculation is required, but two given by the candidate, only the first one will be marked, irrespective of which one is correct. If two answers are required, only the first two will be marked, etc.
3.2 For marking purposes, alternative symbols (s, u, t, etc.) will also be accepted.
3.3 Separate compound units with a multiplication dot, not a full stop, for example, m·s -1. For marking purposes m.s-1and m/s will also be accepted.
4. POSITIVE MARKING
Positive marking regarding calculations will be followed in the following cases:
4.1 Subquestion to subquestion: When a certain variable is incorrectly calculated in one subquestion (e.g. 3.1) and needs to be substituted into another subquestion (3.2 or 3.3), full marks are to be awarded for the subsequent subquestions
4.2 Multi-step question in a subquestion: If the candidate has to calculate, for example, current in the first step and gets it wrong due to a substitution error, the mark for the substitution and the final answer will be forfeited.
5. NEGATIVE MARKING
Normally an incorrect answer cannot be correctly motivated if based on a conceptual mistake. If the candidate is therefore required to motivate in QUESTION 3.2 the answer given to QUESTION 3.1, and 3.1 is incorrect, no marks can be awarded for QUESTION 3.2. However, if the answer for e.g. QUESTION 3.1 is based on a calculation, the motivation for the incorrect answer in QUESTION 3.2 should be considered.
MEMORANDUM
QUESTION 1
1.1 D✔✔ (2)
1.2 C✔✔ (2)
1.3 A✔✔ (2)
1.4 D✔✔ (2)
1.5 B✔✔ (2)
1.6 C✔✔ (2)
1.7 B✔✔ (2)
1.8 A✔✔ (2)
1.9 D✔✔ (2)
1.10 B✔✔ (2)
[20]
QUESTION 2
2.1 0 N/zero✔ (1)
2.2 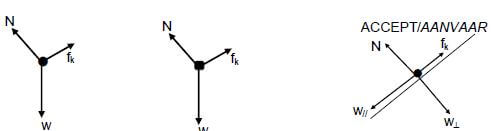
Accepted labels | |
w | Fg/Fw/weight/mg/gravitational force/N/19,6 N |
f | Ff riction/Ff/friction/fk |
N | FN/Fnormal/normal force |
Deduct 1 mark for any additional force. | |
Mark is given for both arrow and label | |
|
2.3.1
- Fnet = ma
fk - mgsinθ = 0
fk = mgsinθ
✔ 1 mark for any of these |
fk = (2)(9,8) sin7º ✔
fk = 2,39 N ✔ (2,389) N (3)
2.3.2 POSITIVE MARKING FROM QUESTION 2.3.1
- fk = μkN
= μkmgcos7º
✔ 1 mark for any of these
2,389 = μk(2)(9,8)cos7º ✔
µk = 0,12 ✔ (3)
2.3.3 POSITIVE MARKING FROM QUESTION 2.3.2
OPTION 1
- Fnet = ma
- fk = ma
- μkN = ma - μk(mg) = ma
✔ 1 mark for any of these
- (0,12)(2)(9,8) ✔= 2a✔
a = -1,176 m.s-2 (-1,18)
vf2 = vi2 + 2aΔx
0 = (1,5)2+ 2(-1,176)Δx✔
Δx = 0,96 m
Distance is 0,96 m✔
OPTION 2
- Wnet = ΔK
Wnet = ΔEK
Wnc = ∆K + ∆U
Wnc = ∆EK + ∆EP
μkN∆x cos θ = ½ mvf2– ½ mvi2
1 mark for any of these
NOTE: substituting into any of the above equations will lead to the following:
(0,12) (2)(9,8) ✔∆x cos 180º ✔= 0 – ½ (2)(1,5)2✔
∆x = 0,957 m✔ (5)
[15]
QUESTION 3
3.1
- (Motion of) an object in which the only force acting is the gravitational force. ✔✔
OR
(Motion of)an object which has been given an initial velocity and which follows a path entirely determined by the effects of gravitational acceleration/force. ✔✔
OR
The (motion of )an object that is projected, thrown or shot either upwards or downwards into the air and on which the only force considered/acting is gravitational. ✔✔ (2)
Note: 2 or/of 0
3.2 No ✔
- The balloon is not accelerating at the rate of 9,8 m∙s-2/moving with constant velocity/acceleration is 0 m∙s-2✔ (2)
OR
There are other forces (e.g.,friction) acting on the balloon besides gravity.✔
Net force acting on the balloon is zero
3.3
OPTION 1 Downward positive |
OPTION 2 vf = vi + aΔt ✔ |
Downward positive vf2 = vi2 + 2aΔy For both equations ✔ vf = vi + aΔt |
OPTION 3 For both equations ✔ Downward positive |
OPTION 4 |
NOTES:
|
(4)
3.4
Upward positive vf = vi + aΔt ✔ OR OR |
Downward Positive vf = vi + aΔt ✔ OR OR |
(6)
[14]
4.1 It is the product of the resultant/net force acting on an object✔ and the time the resultant/net force acts on the object. ✔ (2)
NOTE: ONLY 1 MARK FOR “CHANGE IN MOMENTUM”
4.2.1 (3)
p = mv✔ |
4.2.2
OPTION 1 ∆t for a bullet = 60✔ = 0,27 s OR -77,01 N / - 77,78 N |
OPTION 2 OR - 77,01 N / -77,78 N |
OPTION 3 OR = - 77,01 N/ -77,78 |
NOTE: ACCEPT RANGE: 77 N - 77,78 N |
(5)
4.3
POSITIVE MARKING FROM 4.2.2 77 N/77,78 N✔ to the east✔ |
(2)
[12]
QUESTION 5
5.1 (2)
The rate at which work is done/ Rate at which energy is expended. ✔✔ |
5.2.1 (3)
OPTION 1 |
OPTION 2 | -1 if either negative is omitted or Ep = mgh is used instead of W |
5.2.2 (2)
Wcounterw eight = mg∆ycosθ |
5.3
OPTION 1 -646800✔ + 512050 ✔+ Wmotor = 0 |
OPTION 2 |
OPTION 3 |
(6)
[13]
QUESTION 6
6.1.1 The Doppler effect.✔ (1)
6.1.2 Measuring the rate of blood flow
OR
Ultrasound (scanning)✔ (1)
6.1.3 (6)
FL = V ± VL FS OR FL = V FS OR FL = V FS |
6.1.4
- Increase✔ (1)
- Decrease/✔ (1)
6.2.1 The spectral lines (light) from the star are shifted towards longer wavelengths. ✔✔ (2)
6.2.2 Decrease ✔ (1)
[13]
QUESTION 7
7.1.1 Removed✔ (1)
7.1.2 (3)
n = Q Do not penalise for negative sign of charge used in calculation = 6 × 10-6 |
7.2.1 Negative ✔ (1)
7.2.2 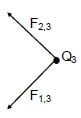 (2)
(2)
NOTE:
Give credit to the required forces even if a triangle of forces is drawn. |
7.2.3 (3)
F = KQ1Q2 |
7.2.4 (4)
POSITIVE MARKING FROM QUESTION 7.2.3 Fx = F1,3x + F2,3x 1 mark for the addition NOTE: Fy net = 0 |
OPTION 2 = (K Q1Q3 )2 + (K Q2Q3)2 |
7.3.1 The electric field at a point is the (electrostatic) force experienced ✔per unit positive charge ✔placed at that point (2)
7.3.2 (5)
OPTION 1 100 = (9×109 )Q When the electric field strength 50 is N∙C-1 E = kQ 50 = (9×109 ) (4 x 10-9) r = 0,85 m (0,845) m✔ |
OPTION 2 E = kQ ∴ E1 = r22 ∴r = 0,85 m (0,849 m)✔ |
[21]
QUESTION 8
NEGATIVE MARKING FOR 8.1.1,8.1.2 AND 8.1.3
8.1.1
- P and Q burn with the same brightness ✔ same potential difference/same current✔ (2)
8.1.2
- P is dimmer (less bright) than R
OR - R is brighter than P✔
R is connected across the battery alone therefore the voltage (terminal pd) is the same as the emf source (energy delivered by the source). ✔
OR
The potential difference across R is twice (larger/greater than) that of P./The current through R is twice (larger/greater than) that of P.
OR
P and Q are in series and are both connected across the same battery, ✔ hence the voltage (terminal pd) is shared equally ✔(P and Q are potential dividers) Therefore R is brighter.
OR
Potential difference across P is half that across R(2)
8.1.3 T does not light up at all✔
ACCEPT
- T is dimmer (less bright) than R✔
- R is brighter than T✔
Reason- The wire acts as a short circuit. ✔
OR - The potential difference across T / current in T is zero.✔ (2)
- The wire acts as a short circuit. ✔
8.2.1 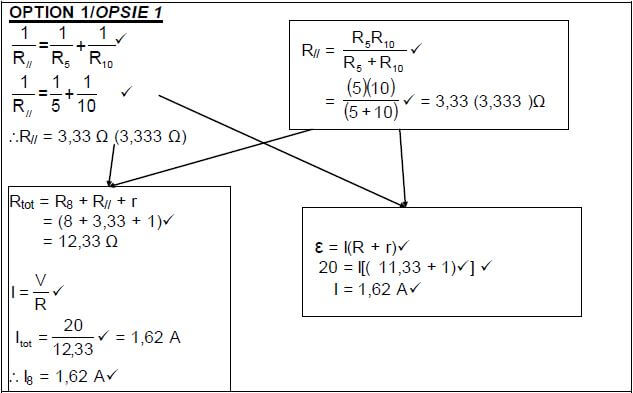
 (6)
(6)
8.2.2
OPTION 1 |
OPTION 2 VR// = R// × Vtot |
OPTION 3 |
(4)
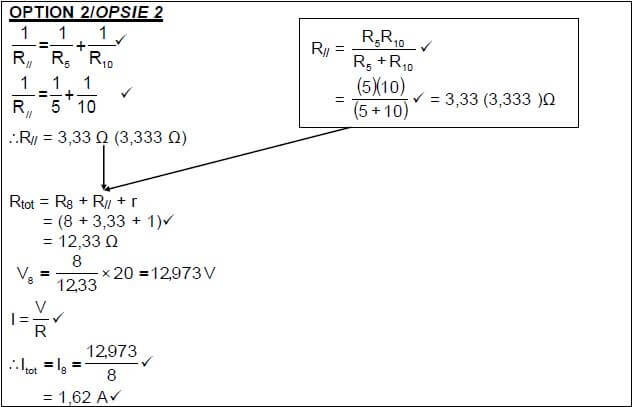
POSITIVE MARKING FROM 8.2.1 I5 = 10 × Itot V5 = I 5 R5 ✔ |
(4)
8.2.3
POSITIVE MARKING FROM 8.2.1 |
POSITIVE MARKING FROM 8.2.1 |
POSITIVE MARKING FROM 8.2.1 AND 8.2.2 |
OPTION 4 | |
P = V2 = 32,44 W✔ | P = I2Rtot ✔ (3) |
NOTE: Range 32,35- 32,45
[19]
QUESTION 9
9.1 Slip rings ✔ (1)
9.2 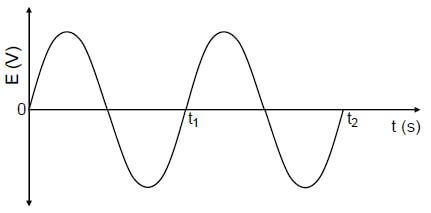 (2)
(2)
Marking criteria | |
Sine graph starts from 0. | ✔ |
Two complete waves (between t0 and t2) | ✔ |
9.3 Any TWO
- Increase the speed of rotation✔
- Increase the number of coils (turns)✔
- Use stronger magnets
ACCEPT: Increase surface area(2)
9.4
- The rms value of an AC voltage it that value of the AC voltage which will dissipate the same amount of energy as DC.
OR - The rms value of an AC voltage it that value of the AC voltage which will produce the same joule heating effect as DC. (2)
9.5 (3)
OPTION 1 1500 = Irms/w gk(240) ✔ Irms/w gk = 1500 | OPTION 2 1500 = 2402 R = 38,4 Ω = 6,25 A✔ |
[10]
QUESTION 10
10.1
- The minimum frequency of light ✔needed to emit electrons from a certain metal surface.✔
OR - The minimum frequency of light✔ below which electrons will not be emitted from the surface of a certain metal. ✔ . (2)
10.2 The speed remains unchanged. ✔ (1)
10.3 (5)
OPTION 1
|
OPTION 2
|
OPTION 3 c = f0λ0✔
|
10.4 (5)
E = Wº + EK(MAX)
Any one of the three ✔ (6,63 x 10-34)(7,8 x 1014) ✔ = (6,63 x 10-34)(6,8 x 1014) + ½mv2max |
[13]
TOTAL: 150
MUSIC PAPER 1 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
MUSIC
PAPER 1
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- This question paper consists of FIVE sections, namely SECTIONS A, B, C, D and E.
- SECTIONS A and B are COMPULSORY.
- SECTION C: WESTERN ART MUSIC (WAM), SECTION D: JAZZ and SECTION E: INDIGENOUS AFRICAN MUSIC (IAM) are choice questions. Answer only ONE of these sections (SECTION C or D or E).
- Write all music notations in SECTION A in pencil and all written text in blue or black ink on this question paper.
- Answer SECTION B and SECTION C or D or E in blue or black ink in the ANSWER BOOK provided.
- Number the questions correctly according to the numbering system used in this question paper.
- The last page of this question paper is manuscript paper intended for rough work. Candidates may remove this page.
- Candidates may NOT have access to any musical instrument for the duration of this examination.
- Candidates must take note of the mark allocation for each question to provide enough information in their answers.
- Write neatly and legibly.
MARKING GRID
SECTION | QUESTION | MARKS | MARKER | MODERATOR |
A: THEORY OF MUSIC (COMPULSORY) | 1 | 20 | ||
2 | 15 | |||
3 | 10 | |||
4 | 15 | |||
SUBTOTAL | 60 | |||
AND | ||||
B: GENERAL MUSIC KNOWLEDGE (COMPULSORY) | 5 | 20 | ||
SUBTOTAL | 20 | |||
AND | ||||
C: WAM | 6 | 10 | ||
7 | 5 | |||
8 | 5 | |||
9 | 5 | |||
10 | 15 | |||
SUBTOTAL | 40 | |||
OR | ||||
D: JAZZ | 11 | 10 | ||
12 | 5 | |||
13 | 5 | |||
14 | 5 | |||
15 | 15 | |||
SUBTOTAL | 40 | |||
OR | ||||
E: IAM | 16 | 10 | ||
17 | 5 | |||
18 | 5 | |||
19 | 5 | |||
20 | 15 | |||
SUBTOTAL | 40 | |||
GRAND TOTAL | 120 | |||
QUESTIONS
SECTION A: THEORY OF MUSIC (COMPULSORY) (90 minutes)
Answer QUESTION 1
AND QUESTION 2.1 OR 2.2
AND QUESTION 3.1 OR 3.2
AND QUESTION 4.1 OR 4.2.
Answer the questions in the spaces provided on this question paper. QUESTION 1 (25 minutes)
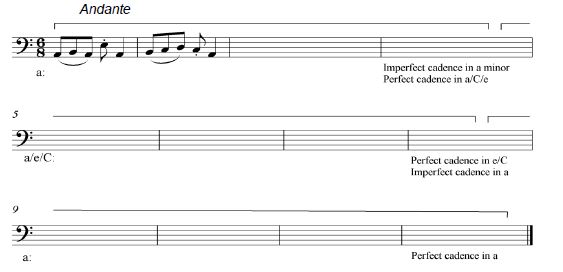
Study the extract below and answer the questions that follow.
Schuber
t
1.1 Name the main key of this piece. ______________________________________________ (1)
1.2 Name the intervals at 1.2.1 and 1.2.2 according to type and distance.
1.2.1 ______________________________________________
1.2.2 ______________________________________________ (2)
1.3 Write and name the inversion of the interval at 1.3. _______________________ (1)
1.4 Name the triads at 1.4.1 and 1.4.2 according to type and position/inversion.
1.4.1 _____________________________________________
1.4.2 ______________________________________________ (2)
1.5 Rewrite bar 1 of the violin part for viola using the same pitch. (2)
1.6 Transpose the bass part from bars 7–10 a perfect fourth higher. Do NOT use a key signature. 
1.7 Rewrite bar 1 of the right-hand part of the piano in compound time. Add the new time signature. 
1.8 Write the scales below as indicated. Use semibreves.
1.8.1 Write F# melodic minor, ascending and descending, with key signature. Mark the semitones. 
1.8.2 Write the Aeolian mode on E, descending in the alto clef.
Do NOT use a key signature. 
1.8.3 Write a chromatic scale on Bb, ascending only. 
[20]
QUESTION 2 (25 minutes)
Answer EITHER QUESTION 2.1 OR QUESTION 2.2.
2.1 Complete the opening motif below to form a twelve-bar melody in ternary form for any single-line melodic instrument of your choice. Name the instrument for which you are writing. Indicate the tempo and add dynamic and articulation marks.
Instrument: _________________________
Tempo: _________________________
The melody will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS |
Form and cadential points | 3 | |
Correctness | 2 | |
Quality | 10 | |
TOTAL | 15 |
[15]
OR
2.2 Complete the opening motif below to form a twelve-bar melody in ternary form for any single-line melodic instrument of your choice. Name the instrument for which you are writing. Indicate the tempo and add dynamic and articulation marks.
Instrument: ________________________
Tempo: _________________________
The melody will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS |
Form and cadential points | 3 | |
Correctness | 2 | |
Quality | 10 | |
TOTAL | 15 |
[15]
QUESTION 3 (10 minutes)
Answer EITHER QUESTION 3.1 OR QUESTION 3.2.
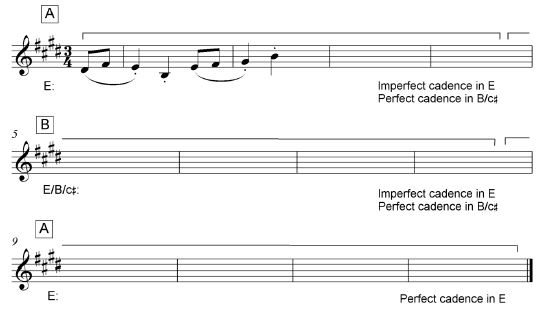
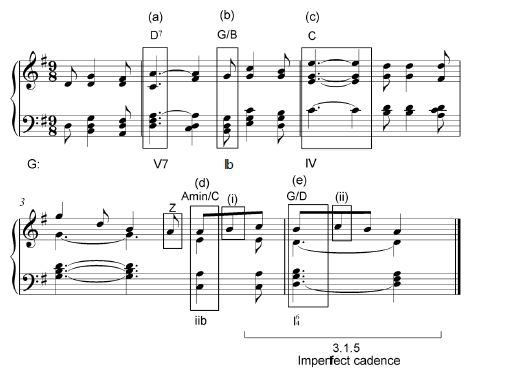
3.1 Study the extract by Mendelssohn below and answer the questions that follow.
3.1.1 Name the key of this extract.
___________________________________________________ (1)
3.1.2 Identify chords (a)–(e) and figure them on the score. Use EITHER figuring below the score, for example V6, OR chord symbols above the score, for example C/E. (5)
3.1.3 Name the types of non-chordal notes at (i) and (ii).
- __________________________________________________
- __________________________________________________ (2)
3.1.4 Which ONE of the following do you associate with the note at Z? Make a cross (X) in the appropriate block. (1)
Anticipation | Suspension | Appoggiatura | Chord note |
3.1.5 Name the cadence with which this extract ends.
_____________________________________________________ (1)
[10]
OR
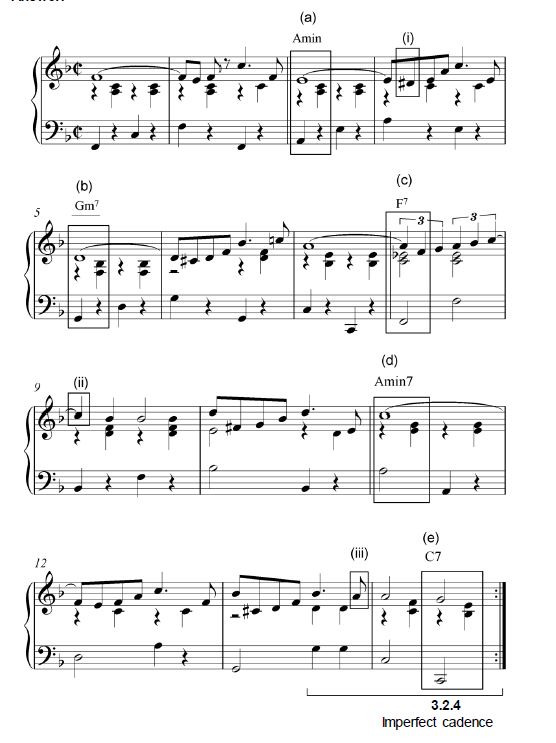
3.2 Study the extract from Till by Charles Danvers below and answer the questions that follow. 
3.2.1 Name the key of this extract.
___________________________________________ (1)
3.2.2 Identify the chords from (a) to (e) and figure them on the score. Use chord symbols above the score, for example Bb/D. (5)
3.2.3 Name the types of non-chordal notes at (i)–(iii).
- _________________________________________________
- _________________________________________________
- _________________________________________________ (3)
3.2.4 Name the cadence at the end of this extract.
__________________________________________________ (1)
[10]
QUESTION 4 (30 minutes)
Answer EITHER QUESTION 4.1 OR QUESTION 4.2.
4.1 Complete the four-part vocal harmonisation below by adding the alto, tenor and bass parts.
The harmonisation will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS |
Chord progression Choice of chords, correct use of cadence | 14 | |
Correctness Notation, doubling, spacing, voice leading | 16 | |
Quality Musicality, non-chordal notes, awareness of style, creativity | 10 | |
40 (÷ 8 x 3) | ||
TOTAL | 15 |
[15]
OR
4.2 Complete the piece below by adding a suitable bass line and harmonic material in the open staves. Continue in the style suggested by the given material in bars 1–4.
The harmonisation will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS |
Chord progression | 15 | |
Correctness | 15 | |
Quality | 10 | |
40 (÷ 8 x 3) | ||
TOTAL | 15 |
[15]
TOTAL SECTION A: 60
SECTIONS B, C, D, E: GENERAL MUSIC KNOWLEDGE (90 minutes)
Answer SECTION B
AND SECTION C (Western Art Music)
OR SECTION D (Jazz)
OR SECTION E (Indigenous African Music).
Answer these questions in the ANSWER BOOK provided.
SECTION B: GENERAL (COMPULSORY)
QUESTION 5
5.1 Various options are provided as possible answers to the following questions. Write down the question number (5.1.1–5.1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE: 5.1.11 |
5.1.1 Royalties paid to composers when their music is performed in public are called …
- copyright.
- performance rights.
- needletime rights.
- mechanical rights.
5.1.2 Royalties paid to songwriters and performers for CD sales or digital downloads are called …
- copyright.
- performance rights.
- needletime rights.
- mechanical rights.
5.1.3 SAMRO is the abbreviation for the …
- South African Music Relevance Organisation.
- South African Music Rights Organisation.
- South African Music Recording Organisation.
- South African Music Restrictions Organisation.
5.1.4 A person who writes the words of a song is a/an …
- arranger.
- editor.
- lyricist.
- performer.
5.1.5 A musical work is copyrighted …
- immediately after it has been composed.
- for 80 years after the work has been composed.
- until the year of the composer's death.
- only two weeks after it has been composed.
5.1.6 The symbol above the given note is called a/an …
- lower mordent.
- appoggiatura.
- upper mordent.
- turn.
5.1.7 In a Dorian mode, semitones occur between the …
- 3rd and 4th notes and the 6th and 7th notes.
- 2nd and 3rd notes and the 7th and 8th notes.
- 3rd and 4th notes and the 7th and 8th notes.
- 2nd and 3rd notes and the 6th and 7th notes.
5.1.8 Which ONE of the following means to become softer gradually?
- Accelerando
- Morendo
- Crescendo
- Rallentando
5.1.9 The musical term for is …
- lungo.
- portato.
- fermata.
- staccato.
5.1.10 The blues scale can be constructed by ….
- lowering the 3rd, 5th and 7th degrees of the major scale.
- raising the 3rd, 5th and 7th degrees of the major scale.
- lowering the 3rd, 5th and 7th degrees of the minor scale.
- raising the 3rd, 5th and 7th degrees of the minor scale. (10 x 1) (10)
5.2 Give the correct term for any FIVE of the following descriptions. Write down only the term next to the question number (5.2.1–5.2.8) in the ANSWER BOOK.
5.2.1 A musical texture consisting of a single melodic line
5.2.2 A musical texture consisting of a melodic line with accompaniment
5.2.3 A musical texture consisting of several independent melodic lines
5.2.4 A rhythmic pattern which repeats while other music material changes around it
5.2.5 Ancient scales with Greek names that are used in various music styles
5.2.6 The quality or colour of a voice or an instrument
5.2.7 Vocal music without instrumental accompaniment
5.2.8 The technical name for the third degree of a scale (5)
5.3 Write a paragraph in which you describe binary form. (5)
TOTAL SECTION B: 20
Answer SECTION C (WAM)
OR SECTION D (JAZZ)
OR SECTION E (IAM).
SECTION C: WESTERN ART MUSIC (WAM)
QUESTION 6
6.1 Which characters are associated with the following voice types in The Magic Flute by Mozart?
6.1.1 Soprano
6.1.2 Tenor
6.1.3 Baritone
6.1.4 Bass
6.1.5 Coloratura (5)
6.2 The Magic Flute is considered to be a Singspiel.
Write notes to substantiate this statement using examples from this opera. (5)
[10]
QUESTION 7
Study the table below which represents sonata form and answer the questions that follow.
EXPOSITION | DEVELOPMENT | RECAPITULATION |
7.1 In which section would one expect to find an episode? (1)
7.2 What is the function of the bridge in the exposition of this form? (1)
7.3 Briefly describe what happens in the development section. (2)
7.4 How is the recapitulation different from the exposition? (1)
[5]
QUESTION 8
Write a paragraph on the final (fifth) movement of Beethoven's Symphony No. 6 in which you link the title of this movement to the programmatic content. [5]
QUESTION 9
Define the Classical symphony and expain how Beethoven's Pastoral Symphony differs from the Classical symphonic model. [5]
QUESTION 10
Mendelssohn demonstrates both Classical and Romantic features in his Hebrides Overture.
Write an essay in which you discuss this statement.
You will be credited for the logical presentation of facts and the structure of your essay. The essay will be marked according to the following criteria:
CRITERIA | MARK ALLOCATION |
Classical features | 6 |
Romantic features | 6 |
Logical presentation and structure of the essay | 3 |
TOTAL | 15 |
[15]
TOTAL SECTION C: 40
OR
SECTION D: JAZZ
QUESTION 11
11.1 Describe prominent music characteristics of kwela. (3)
11.2 Name the instruments used in a typical mbaqanga band. (3)
11.3 Write down the title of a song associated with EACH of the following artists/groups:
11.3.1 Dolly Rathebe
11.3.2 Miriam Makeba
11.3.3 Sakhile
11.3.4 Philip Tabane (4)
[10]
QUESTION 12
Indicate whether the following statements concerning Cape jazz are TRUE or FALSE. Write down only 'true' or 'false' next to the question number (12.1–12.5) in the ANSWER BOOK.
12.1 It is inspired by blues and folk songs sung by descendants of the former slave communities living in the Western Cape.
12.2 It is influenced by the street carnival parade and instrumentation of the Mardi Gras.
12.3 It is a mixture of Xhosa and Zulu songs, as well as Latin-American styles.
12.4 It was originally mainly a piano jazz style.
12.5 Robbie Jansen is a famous saxophone player who is linked to the development of Cape jazz.
[5]
QUESTION 13
Discuss TWO international influences and TWO local influences on Miriam Makeba's music style. Comment on her unique vocal style. [5]
QUESTION 14
Write a paragraph in which you discuss the importance of the Jazz Epistles in the development of South African jazz. [5]
QUESTION 15
Marabi is a true example of an early South African jazz style.
Write an essay in which you expand on this statement by refering to the origins, characteristics and music examples of marabi.
You will be credited for the logical presentation of facts and the structure of your essay. The essay will be marked according to the following criteria:
CRITERIA | MARK ALLOCATION |
Origins | 5 |
Characteristics | 5 |
Music examples | 2 |
Logical presentation and structure of the essay | 3 |
TOTAL | 15 |
[15]
TOTAL SECTION D: 40
OR
SECTION E: INDIGENOUS AFRICAN MUSIC (IAM)
QUESTION 16
16.1 Indicate whether the following statements are TRUE or FALSE. Write only 'true' or 'false' next to the question number (16.1.1–16.1.4) in the ANSWER BOOK. If the statement is FALSE, write down the correct information.
16.1.1 Isicathamiya was popularised internationally by Ladysmith Black Mambazo's collaboration with Simon and Garfunkel.
16.1.2 Julian Bahula played drums for Sello Galane.
16.1.3 Mahotella Queens is a group that sings free kiba.
16.1.4 The Manhattan Brothers was a kwela group. (4)
16.2 Define malombo. (2)
16.3 Define the following terms associated with maskandi:
16.3.1 Ikati
16.3.2 Ukupika (4)
[10]
QUESTION 17
Write a paragraph in which you define and describe free kiba. Refer to the traditional drums used in this style of music. [5]
QUESTION 18
Briefly discuss ONE of the following ceremonies. Refer to the function, ceremonial features and the role of dance, music and instruments.
- AmaZulu: Amahubo
- AmaSwati: Incwala
- AmaXhosa: Intonjane
- AmaNdebele: Luma
- Basotho: Lebollo
- Bapedi: Byale
- Batswana: Bojale
- Vhavenda: Domba
- Batsonga: Mancomane [5]
QUESTION 19
Discuss the features of praise poetry as used in African music. [5]
QUESTION 20
Isicathamiya has become one of the most readily recognised South African music genres of the late 20th and early 21st centuries.
Write an essay in which you discuss this statement with specific reference to Ladysmith Black Mambazo.
You will be credited for the logical presentation of facts and the structure of your essay.
The essay will be marked according to the following criteria:
CRITERIA | MARK ALLOCATION |
Origins | 3 |
Style characteristics | 5 |
Contribution of Ladysmith Black Mambazo | 4 |
Logical presentation and structure of the essay | 3 |
TOTAL | 15 |
[15]
TOTAL SECTION E: 40
GRAND TOTAL: 120
MUSIC PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
MUSIC
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- This question paper consists of THREE sections:
SECTION A: Aural (10)
SECTION B: Recognition (12)
SECTION C: Form (8) - QUESTION 1, QUESTION 2, QUESTION 3 and QUESTION 7 are COMPULSORY.
- Answer QUESTION 4: Western Art Music (WAM) OR QUESTION 5: Jazz OR QUESTION 6: Indigenous African Music (IAM).
- Write ALL your answers on this question paper. Use a pencil for music notation and blue or black ink for the other answers.
- This examination will be written while candidates are listening to a CD. 6.
- The music teacher of the centre must conduct the examination in the presence of the invigilator.
- The last page of this question paper is manuscript paper intended for rough work. The candidate MAY NOT remove this page.
- Candidates may NOT have access to any musical instrument for the duration of this examination.
- Candidates must take note of the mark allocation at each question to provide enough information in their answers.
- Write neatly and legibly.
INSTRUCTIONS TO THE PERSON OPERATING THE SOUND EQUIPMENT
|
SUMMARY OF MARKS
SECTION A: AURAL | TOTAL |
QUESTION 1 (COMPULSORY) | 4 |
QUESTION 2 (COMPULSORY) | 6 |
SUBTOTAL | 10 |
SECTION B: RECOGNITION | TOTAL |
QUESTION 3 (COMPULSORY) | 4 |
QUESTION 4 (WAM) OR | 8 |
QUESTION 5 (JAZZ) OR | 8 |
QUESTION 6 (IAM) | 8 |
SUBTOTAL | 12 |
SECTION C: FORM | TOTAL |
QUESTION 7 (COMPULSORY) | 8 |
SUBTOTAL | 8 |
GRAND TOTAL | 30 |
QUESTIONS
SECTION A: AURAL
QUESTION 1
Play Track 1 TWICE in succession. |
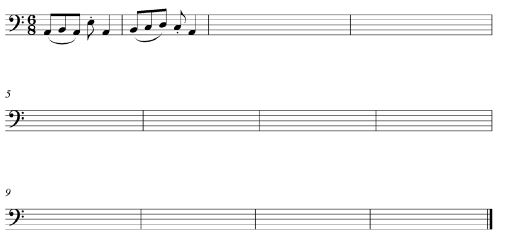
1.1 Listen to the melodic and rhythmic phrase. Notate the rhythm of the missing notes in bars 3–4 below.
![]() (3)
(3)
Play Track 1 TWICE again. |
Play Track 2 THREE times in succession. |
1.2 Which ONE of the extracts below best represents the solo violin part? Make a cross (X) in the appropriate block.  (1)
(1)
[4]
Play Track 2 ONCE more. |
QUESTION 2
Play Track 3 ONCE to provide a general overview. |
Listen to the extract from Menuet in G by Mozart. Answer the questions that follow.
Play Track 4 TWICE. |
2.1 The notation of bars 23b–42 has been omitted at 2.1 in the score. Fill in the missing pitches on the score that correspond to the music that you hear. (3)
Play Track 5 ONCE. |
2.2 Name the cadence at (a) in bars 73–82. (The track starts in bar 1.) (1)
Play Track 6 ONCE. |
2.3 Listen to the music in bars 83–122. Which compositional technique is used at (b)? (The track starts in bar 83.) (1)
Play Track 7 TWICE. |
2.4 Complete the missing bass notes at (c) on the score. (The track starts in bar 123.) (1)
[6]
Play Track 8 for a final overview. |
TOTAL SECTION A: 10
SECTION B: RECOGNITION OF MUSIC CONCEPTS
QUESTION 3: GENERAL LISTENING (COMPULSORY)
Listen to the following tracks and answer the questions that follow.
Play Track 9 ONCE. |
3.1 Listen to the music and indicate ONE feature that you hear. Make a cross (X) in the appropriate block.
Pesante | Orchestra | Pizzicato | String ensemble |
(1)
Play Track 10 TWICE. |
3.2 Choose any ONE item in COLUMN A and briefly describe what you hear in COLUMN B.
COLUMN A | COLUMN B DESCRIPTION |
Tonality | |
Vocal technique | |
Voice type |
(1)
Play Track 11 TWICE. |
3.3 Choose any TWO items in COLUMN A and briefly describe what you hear in COLUMN B.
COLUMN A | COLUMN B DESCRIPTION |
Compositional technique | |
Harmony | |
Time signature |
(2)
Play Tracks 12 and 13 in succession. |
3.4 In Tracks 12 and Track 13 you will hear TWO different performances of the same piece. Compare these two extracts in terms of the following:
ELEMENT | COMPARISON | |
Track 12 | Track 13 | |
Instrumentation | ||
Texture | ||
Style | ||
Use of rhythm | ||
(4)
(8 ÷ 2) [4]
Answer QUESTION 4 (WAM) OR QUESTION 5 (JAZZ) OR QUESTION 6 (IAM).
QUESTION 4: WAM
4.1 Listen to the following extract and answer the questions that follow.
Play Track 14 ONCE. |
4.1.1 Name the work from which this extract has been taken.
____________________________________________________ (1)
4.1.2 Identify the tonality of this extract. Make a cross (X) in the appropriate block.
Chromatic | Modal | Major |
(1)
Play Track 15 ONCE. |
4.1.3 Describe the melodic line. (2)
4.1.4 Identify the cadence at the end of this extract. (1)
4.2 Listen to the extract from Mozart's The Magic Flute in Track 16 and answer the questions that follow.
Play Track 16 TWICE. |
4.2.1 Name the character who sings in this extract. (1)
4.2.2 What does this character represent in the opera? (1)
4.2.3 Name the voice type that you hear in this extract. (1)
4.2.4 Suggest a suitable Italian tempo indication for this extract. (1)
4.2.5 Where in the opera is this extract sung? (1)
4.2.6 Describe what is happening in the storyline at this point. (1)
4.3 Listen to the extract from Beethoven's Symphony No. 6 in Track 17 and answer the questions that follow.
Play Track 17 ONCE. |
4.3.1 Choose the term that refers to this extract. Make a cross (X) in the appropriate block. (1)
Tutti | Ostinato | Attacca | Melisma |
Play Track 18 ONCE. |
4.3.2 Name the woodwind instrument which plays the high-pitched melodic fragment in this extract. (1)
4.3.3 From which movement of the Symphony No. 6 by Beethoven has this extract been taken? (1)
Play Track 19 TWICE. |
4.4 Describe TWO style characteristics that you hear in this extract. (2)
(16 ÷ 2) [8]
TOTAL SECTION B: 12
OR
QUESTION 5: JAZZ
5.1 Listen to the extracts below and answer the questions that follow.
Play Track 20 TWICE. |
5.1.1 With which of the following styles would you associate this extract? Make a cross (X) in the appropriate block. (1)
Kwela | Cape jazz | Modern jazz |
5.1.2 Identify the piece in this extract. (1)
5.1.3 Name TWO artists that are associated with the music style in this extract. (2)
5.1.4 Identify TWO idiophones that are part of the rhythm section in the music in this extract. (2)
5.2 Listen to the Track 21 and answer the questions that follow.
Play Track 21 TWICE. |
5.2.1 Identify the jazz style in this extract. (1)
5.2.2 Give reasons, related to the music, for your answer to QUESTION 5.2.1. (3)
5.2.3 Which jazz artist from the 1950s had an influence on this type of jazz? (1)
5.3 Listen to Track 22 and answer the questions that follow.
Play Track 22 TWICE. |
5.3.1 Give reasons why you would regard the extract as a typical Cape jazz piece. (3)
Play Track 23 ONCE. |
5.3.2 Describe the style of saxophone-playing in your own words. (2)
(16 ÷ 2) [8]
OR
QUESTION 6: IAM
6.1 Listen to the extracts below and answer the questions that follow.
Play Track 24 ONCE. |
6.1.1 Identify the style of music in this extract. (1)
Play Track 25 THREE times in succession. |
6.1.2 Choose the order in which the instruments appear. Make a cross (X) in the appropriate block. (1)
INSTRUMENT ORDER | |
Drums/Percussion, piano, bass guitar | |
Drums/Percussion, bass guitar, piano | |
Drums/Percussion, piano and bass guitar |
Play Track 26 ONCE. |
6.1.3 What is the role of the female voices in this song? (1)
6.1.4 What typical African compositional technique is heard in this extract? (1)
6.2 Listen to the following TWO tracks which will be played in succession and answer the questions that follow.
Play Track 27 and Track 28 ONCE in succession. |
6.2.1 The TWO extracts have a similar purpose or function. Explain the purpose or function of the music in these two extracts. (2)
6.2.2 Indicate ONE group associated with Track 27. Make a cross (X) in the appropriate block. (1)
ZCC | Amazayoni | Apostolic Church | Shembe |
Play Track 29 ONCE. |
6.2.3 Describe the use of rhythm in the membranophones. (1)
Play Track 30 ONCE. |
6.2.4 Describe the texture of this song. (1)
6.3 Listen to the extract below and answer the questions that follow.
Play Track 31 THREE times. |
6.3.1 With what indigenous South African music style would you associate this extract? (1)
6.3.2 Which music performance characteristics heard in this extract, are common to the style of music in QUESTION 6.3.1? (3)
Play Track 32 ONCE. |
6.4 Identify the style of music in this extract. Give TWO reasons for your answer.
Style:
Reasons: (3)
(16 ÷ 2) [8]
TOTAL SECTION B: 12
SECTION C: FORM
QUESTION 7
Read and study the questions for ONE minute.
Play Track 33 ONCE to provide an overview. |
Listen to the following piece while you study the score.
Play Track 33 again. |
7.1 What is the overall form of this piece? (1)
7.2 Motivate your answer to QUESTION 7.1 by giving a schematic layout of the form of this piece. Use the table below. (3)
SECTION | BAR NUMBERS |
7.3 Name the key of this piece. (1)
7.4 Choose the term that describes the mood of this piece. Make a cross (X) in the appropriate block. (1)
Affettuoso | Giocoso | Maestoso | Mosso |
Play Track 34 TWICE. |
7.5 Which compositional technique is used in bars 31–32? (The track starts in bar 31.) (1)
Play Track 35 TWICE. |
7.6 Write down an Italian term which describes what happens to the tempo in bar 34. (The track starts in bar 33.) (1)
[8]
Play Track 36 for a final overview. |
TOTAL SECTION C: 8
GRAND TOTAL: 30
MUSIC PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
MUSIC
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- This question paper consists of THREE sections:
SECTION A: Aural (10)
SECTION B: Recognition (12)
SECTION C: Form (8) - QUESTION 1, QUESTION 2, QUESTION 3 and QUESTION 7 are COMPULSORY.
- Answer QUESTION 4: Western Art Music (WAM) OR QUESTION 5: Jazz OR QUESTION 6: Indigenous African Music (IAM).
- Write ALL your answers on this question paper. Use a pencil for music notation and blue or black ink for the other answers.
- This examination will be written while candidates are listening to a CD. 6.
- The music teacher of the centre must conduct the examination in the presence of the invigilator.
- The last page of this question paper is manuscript paper intended for rough work. The candidate MAY NOT remove this page.
- Candidates may NOT have access to any musical instrument for the duration of this examination.
- Candidates must take note of the mark allocation at each question to provide enough information in their answers.
- Write neatly and legibly.
INSTRUCTIONS TO THE PERSON OPERATING THE SOUND EQUIPMENT
|
SUMMARY OF MARKS
SECTION A: AURAL | TOTAL |
QUESTION 1 (COMPULSORY) | 4 |
QUESTION 2 (COMPULSORY) | 6 |
SUBTOTAL | 10 |
SECTION B: RECOGNITION | TOTAL |
QUESTION 3 (COMPULSORY) | 4 |
QUESTION 4 (WAM) OR | 8 |
QUESTION 5 (JAZZ) OR | 8 |
QUESTION 6 (IAM) | 8 |
SUBTOTAL | 12 |
SECTION C: FORM | TOTAL |
QUESTION 7 (COMPULSORY) | 8 |
SUBTOTAL | 8 |
GRAND TOTAL | 30 |
Note to the marker: Candidates must be credited for any correct answers not given in the memorandum. |
MEMORANDUM
SECTION A: AURAL
QUESTION 1
Play Track 1 TWICE in succession. |
1.1 Listen to the melodic and rhythmic phrase. Notate the rhythm of the missing notes in bars 3–4 below.
Play Track 1 TWICE again. |
Answer: 
½ mark per note as indicated = 3 marks |
Play Track 2 THREE times in succession. |
1.2 Which ONE of the extracts below best represents the solo violin part? Make a cross (X) in the appropriate block.
 (1)
(1)
[4]
Play Track 2 ONCE more. |
Correct answer = 1 mark |
QUESTION 2
Play Track 3 ONCE to provide a general overview. |
Listen to the extract from Menuet in G by Mozart. Answer the questions that follow.
Play Track 4 TWICE. |
2.1 The notation of bars 23b–42has been omitted at 2.1 in the score. Fill in the missing pitches on the score that correspond to the music that you hear.
Answer: 
½ mark for each correct pitch = 6 X ½= 3 marks |
Play Track 5 ONCE. |
2.2 Name the cadence at (a) in bars 73–82. (The track starts in bar 1.)
Answer:
- Perfect (cadence) (1)
1 mark |
Play Track 6 ONCE. |
2.3 Listen to the music in bars 83–122. Which compositional technique is used at (b)? (The track starts in bar 83.)
Answer:
- Sequence
- Rhythmic repetition (1)
Any correct answer = 1 mark |
Play Track 7 TWICE. |
2.4 Complete the missing bass notes at (c) on the score. (The track starts in bar 123.)
Answer: (1)
½ mark for each correct pitch = 1 mark |
[6]
Play Track 8 for a final overview. |
TOTAL SECTION A: 10
SECTION B: RECOGNITION OF MUSIC CONCEPTS
QUESTION 3: GENERAL LISTENING (COMPULSORY)
Listen to the following tracks and answer the questions that follow.
Note to marker: if a candidate selected more than one item in a question, only the first item must be marked. |
Play Track 9 ONCE. |
3.1 Listen to the music and indicate ONE feature that you hear. Make a cross (X) in the appropriate block.
Answer:
- String ensemble (1)
1 mark |
Play Track 10 TWICE. |
3.2 Choose any ONE item in COLUMN A and briefly describe in COLUMN B what you hear.
Answer:
COLUMN A | COLUMN B DESCRIPTION |
Tonality | Major |
Vocal technique | Scatting (vocal nonsense-syllable singing)/ vocal improvisation/vocalise |
Voice type | Female voice/alto |
Correct answer = 1 mark |
(1)
Play Track 11 TWICE. |
3.3 Choose any TWO items in COLUMN A and briefly describe in COLUMN B what you hear.
Answer:
COLUMN A | COLUMN B DESCRIPTION |
Compositional technique | Call and response/Imitation/Repetition |
Harmony | Repeated pattern |
Time signature | 4/4 or 2/2 |
Correct answers = 2 marks |
(2)
Note to marker: if a candidate selected more than two items, only the first two items must be marked. |
Play Tracks 12 and 13 in succession. |
3.4 In Tracks 12 and 13 you will hear TWO different performances of the same piece. Compare these two extracts in terms of the following:
Answer: (4)
Track 12 | Track 13 | |
Instrumentation | Harpsichord | Piano, drums, bass |
Texture | Polyphonic | Polyphonic |
Style | Baroque | Jazz |
Use of Rhythm | Straight | Swing |
1 correct answer per comparison = 1 mark No ½ marks |
(8 ÷2) (4)
TOTAL SECTION B: 4
Answer QUESTION 4 (WAM) OR QUESTION 5 (JAZZ) OR QUESTION 6 (IAM).
QUESTION 4: WAM
4.1 Listen to the following extract and answer the questions that follow.
Play Track 14 ONCE. |
4.1.1 Name the work from which this extract has been taken.
Answer:
- Hebrides Overture
- Fingal's Cave (1)
Any correct answer = 1 mark |
4.1.2 Identify the tonality of this extract. Make a cross (X) in the appropriate block.
Answer:
- Major (1)
1 mark |
Play Track 15 ONCE. |
4.1.3 Describe the melodic line.
Answer:
- Legato/Smooth/Flowing
- Cantabile/in a singing style/Lyrical
- Rapid crescendos and decrescendos
- Constant ascending and descending line
- Played by cello section (2)
Any 2 correct answers = 2 marks |
4.1.4 Identify the cadence at the end of this extract.
Answer:
- Perfect cadence (1)
1 mark |
4.2 Listen to the extract from Mozart's The Magic Flute in Track 16 and answer the questions that follow.
Play Track 16 TWICE. |
4.2.1 Name the character who sings in this extract.
Answer:
- Sarastro (1)
1 mark |
4.2.2 What does this character represent in the opera?
Answer:
- Goodness
- Fatherhood
- Reverence (1)
Any correct answer = 1 mark |
4.2.3 Name the voice type that you hear in this extract.
Answer:
- Bass (1)
1 mark |
4.2.4 Suggest a suitable Italian tempo indication for this extract.
Answer:
- Adagio
- Largo (1)
Any 1 suitable Italian term = 1 mark |
4.2.5 Where in the opera is this extract sung?
Answer:
- Act 2
- Before Tamino is subjected to the trials (1)
Any correct answer = 1 mark |
4.2.6 Describe what is happening in the storyline at this point.
Answer:
- Sarastro sings of the ideals of the Brotherhood after Pamina pleads for mercy for her mother, the Queen
- Sarastro makes a plea to the Egyptian gods to assist Tamino during his trials ∙ (1)
Any correct answer = 1 mark |
4.3 Listen to the extract from Beethoven's Symphony No. 6 in Track 17 and answer the questions that follow.
Play Track 17 ONCE. |
4.3.1 Choose the term that refers to this extract. Make a cross (X) in the appropriate block.
Answer:
- Tutti (1)
1 mark |
Play Track 18 ONCE. |
4.3.2 Name the woodwind instrument which plays the high-pitched melodic fragment in this extract.
Answer:
- Flute (1)
1 mark |
4.3.3 From which movement of the Symphony No. 6 by Beethoven has this extract been taken?
Answer:
- First movement (1)
1 mark |
Play Track 19 TWICE. |
4.4 Describe TWO style characteristics that you hear in this extract.
Answer:
- Homophonic texture
- Regular phrasing
- Clear-cut cadences
- Melodic motifs used abundantly
- Concerto genre used (oboe and strings) (2)
Any 2 correct answers = 2 marks |
(16 ÷ 2) [8]
TOTAL SECTION B: 12
OR
QUESTION 5: JAZZ
5.1 Listen to the extracts and answer the questions that follow.
Play Track 20 TWICE. |
5.1.1 With which of the following styles would you associate this extract? Make a cross (X) in the appropriate block.
Answer:
- Modern jazz (1)
1 mark |
5.1.2 Identify the piece in this extract
Answer:
- Shebeen (by Hugh Masekela & The Union of South Africa) (1)
1 mark |
5.1.3 Name TWO artists that are associated with the music style in this extract.
Answer:
- Hugh Masekela (Trumpet)
- Jonas Gwangwa (Trombone)
- Caiphus Semenya (Alto saxophone)
Any 2 correct artists = 2 marks |
OR
- Hugh Masekela and The Union of South Africa (2)
Answer = 2 marks |
5.1.4 Identify TWO idiophones that are part of the rhythm section in the music in this extract.
Answer:
- Shakers
- Cow bell
- Cymbals
- Tambourine (2)
Any 2 correct answers = 2 marks |
5.2 Listen to the extract in Track 21 and answer the questions that follow.
Play Track 21 TWICE. |
5.2.1 Identify the jazz style in this extract.
Answer:
- Kwela (1)
1 mark |
5.2.2 Give reasons, related to the music, for your answer to QUESTION 5.2.1.
Answer:
- Cyclic chord structure
- Solo player with band
- Lively tempo
- Skiffle-like beat
- Jive/Swing rhythms
- Melodic material developed in improvisation
- Pennywhistle (3)
Any 3 correct answers = 3 marks |
5.2.3 Which jazz artist from the 1950s had an influence on this type of jazz?
Answer:
- Spokes Mashiyane
- Lemmy Mabaso
- Elias Lerole (1)
Any 1 correct artist = 1 mark |
5.3 Listen to the extract in Track 22 and answer the questions that follow.
Play Track 22 TWICE. |
5.3.1 Give reasons why you would regard the extract as a typical Cape jazz piece.
Answer:
- Rhythmic characteristics of amaXhosa music
- Ghoema beat
- Marching and Christmas band harmonies
- Banjo and guitar rhythms from Kaapse Klopse
- Specific playing style of piano
- Saxophone produces melody in a nasal tone with vibrato at the end of phrases
- Characteristics of Khoi-San mouth bow evident (3)
1 mark for each correct answer up to 3 marks |
Play Track 23 ONCE. |
5.3.2 Describe the style of saxophone playing in your own words.
Answer:
- It plays the melody
- Uses a scooping performance technique
- Plays in the middle register
- Nasal tone colour used
- Vibrato at the end of phrases (2)
Any 2 correct answers = 2 marks |
(16 ÷ 2) [8]
TOTAL SECTION B: 12
OR
QUESTION 6: IAM
6.1 Listen to the extracts and answer the questions that follow.
Play Track 24 ONCE. |
6.1.1 Identify the style of music in this extract.
Answer:
- Free Kiba
- Malombo
- African Jazz (1)
Any correct answer = 1 mark |
Play Track 25 THREE times in succession. |
6.1.2 Choose the order in which the instruments appear. Make a cross (X) in the appropriate block.
Answer:
Instrument order | |
Drums/Percussion, piano, bass guitar | |
Drums/Percussion, bass guitar, piano | x |
Drums/Percussion, piano and bass guitar |
Correct answer = 1 mark |
(1)
Play Track 26 ONCE. |
6.1.3 What is the role of the female voices in this song?
Answer:
- Backing vocals
- Response to the 'call' of leader (male)
- Fills in the harmony (1)
Any correct answer = 1 mark |
6.1.4 What typical African compositional technique is heard in this extract?
Answer:
- Call and response (1)
1 mark |
6.2 Listen to the following TWO tracks which will be played in succession and answer the questions that follow.
Play Track 27 and Track 28 ONCE in succession. |
6.2.1 The TWO extracts have a similar purpose or function. Explain the purpose and function of the music in these two extracts.
Answer:
- Religious or sacred purpose
- Ritualistic
- Communication with Ancestors/God
- Relate to African divinity (2)
Any 2 correct answers = 2 marks |
6.2.2 Indicate ONE group associated with Track 27. Make a tick (X) in the appropriate block.
ZCC | Amazayoni | Apostolic Church | Shembe |
Answer:
- Amazayoni (1)
1 mark |
Play Track 29 ONCE. |
6.2.3 Describe the use of rhythm in the membranophones.
Answer:
- Polyrhythms
- Syncopation
- Repetitive (1)
Any correct answer = 1 mark |
Play Track 30 ONCE. |
6.2.4 Describe the texture of this song.
Answer:
- Overlapping voices
- Call and response (1)
Any correct answer = 1 mark |
Play Track 31 THREE times. |
6.3 Listen to the extract below and answer the questions that follow.
6.3.1 With what indigenous South African music style would you associate this extract?
Answer:
- Isicathamiya (1)
1 mark |
6.3.2 Which music performance characteristics heard in this extract, are common to the style of music in 6.3.1?
Answer:
- Overlapping voices
- Male choir/ensemble
- Use of falsetto
- Repetitive cyclic harmonic progressions (3)
1 mark for each correct answer up to a maximum of 3 marks |
Play Track 32 ONCE. |
6.4 Identify the style of music in this extract. Give TWO reasons for your answers.
Answer:
- Style: Mbaqanga
Reasons:
- Small ensemble of players
- Use of repetitive guitar melodic riffs
- Interweaving electric guitar lines repeated throughout
- Guitar introduction
- Guitar strings have a soft tone quality on electric guitar
- R&B fused with the cyclic structure of Marabi
- Emphasis on off-beats typical of the style (3)
Mbaqanga = 1 mark Reasons: Any 2 correct answers = 2 marks |
(16 ÷ 2) [8]
TOTAL SECTION B: 12
SECTION C: FORM
QUESTION 7
Read and study the questions for ONE minute.
Play Track 33 ONCE to provide an overview. |
Listen to the following piece while you study the score.
Play Track 33 again. |
7.1 What is the overall form of this piece?
Answer:
- Ternary form
- ABA (1)
Any correct answer = 1 mark |
7.2 Motivate your answer to QUESTION 7.1 by giving a schematic layout of the form of this piece. Use the table below.
Answer:
Section | Bar numbers | Marks |
A ½ (including Introduction) or A ½ | 1–20 ½ or 5–20 ½ | = 1 mark |
B ½ | 21–34 ½ | = 1 mark |
A ½ | 35–52 ½ | = 1 mark |
Cadence extension (part of A) | 50–52 |
½ mark for each correct section = 1½ marks ½ mark for each correct set of bar numbers = 1½ marks |
(3)
7.3 Name the key of this piece.
Answer:
- A minor (1)
1 mark |
7.4 Choose the term that describes the mood of this piece. Make a cross (X) in the appropriate block.
Answer:
- Affettuoso (1)
1 mark |
Play Track 34 TWICE. |
7.5 Which compositional technique is used in bars 31–32? (The track starts in bar 31.)
Answer:
- Imitation/Repetition of a three- note motive an octave higher (1)
1 mark |
Play Track 35 TWICE. |
7.6 Write down an Italian term which describes what happens to the tempo in bar 34. (The track starts in bar 33.)
Answer:
- Ritardando
- Rallentando (1)
Any correct answer = 1 mark |
[8]
Play Track 36 for a final overview. |
TOTAL SECTION C: 8
GRAND TOTAL: 30
MUSIC PAPER 1 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
MUSIC
PAPER 1
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- This question paper consists of FIVE sections, namely SECTIONS A, B, C, D and E.
- SECTIONS A and B are COMPULSORY.
- SECTION C: WESTERN ART MUSIC (WAM), SECTION D: JAZZ and SECTION E: INDIGENOUS AFRICAN MUSIC (IAM) are choice questions. Answer only ONE of these sections (SECTION C or D or E).
- Write all music notation in SECTION A in pencil and all written text in blue or black ink on this question paper.
- Answer SECTION B and SECTION C or D or E in blue or black ink in the ANSWER BOOK provided.
- Number the answers correctly according to the numbering system used in this question paper.
- The last page of this question paper is manuscript paper intended for rough work. Candidates may remove this page.
- Candidates may NOT have access to any musical instrument for the duration of this examination.
- Candidates must take note of the mark allocation for each question to provide enough information in their answers.
- Write neatly and legibly.
MEMORANDUM
MARKING GRID
SECTION | QUESTION | MARKS | MARKER | MODERATOR |
A: THEORY OF MUSIC (COMPULSORY) | 1 | 20 | ||
2 | 15 | |||
3 | 10 | |||
4 | 15 | |||
SUBTOTAL | 60 | |||
AND | ||||
B: GENERAL (COMPULSORY) | 5 | 20 | ||
SUBTOTAL | 20 | |||
AND | ||||
C: WAM | 6 | 10 | ||
7 | 5 | |||
8 | 5 | |||
9 | 5 | |||
10 | 15 | |||
SUBTOTAL | 40 | |||
OR | ||||
D: JAZZ | 11 | 10 | ||
12 | 5 | |||
13 | 5 | |||
14 | 5 | |||
15 | 15 | |||
SUBTOTAL | 40 | |||
OR | ||||
E: IAM | 16 | 10 | ||
17 | 5 | |||
18 | 5 | |||
19 | 5 | |||
20 | 15 | |||
SUBTOTAL | 40 | |||
GRAND TOTAL | 120 | |||
SECTION A: THEORY OF MUSIC (COMPULSORY) (90 minutes)
Answer QUESTION 1
AND QUESTION 2.1 OR 2.2
AND QUESTION 3.1 OR 3.2
AND QUESTION 4.1 OR 4.2.
Answer the questions in the spaces provided on this question paper.
QUESTION 1 (25 minutes)
Study the extract below and answer the questions that follow.
Schuber
t
1.1 Name the main key of this piece.
Answer:
- D minor (1)
D minor = 1 mark |
1.2 Name the intervals at 1.2.1 and 1.2.2 according to type and distance. Answer:
1.2.1 Compound major 3rd/Major 10th
1.2.2 Minor 6th (2)
Compound major 3rd/Major 10th = 1 mark |
1.3 Write and name the inversion of the interval at 1.3.
Answer:
- Minor 7th (1)
Minor 7th= ½ mark |
1.4 Name the triads at 1.4.1 and 1.4.2 according to type and position/inversion.
Answer:
1.4.1 Minor – second inversion
1.4.2 Major – root position (2)
Minor = ½ mark |
1.5 Rewrite bar 1 of the violin part for viola using the same pitch.
½ ½ 
Clef = 1 mark |
1.6 Transpose the bass part from bars 7–10 a perfect fourth higher. Do NOT use a key signature.
Answer: 
Notation = ½ mark per bar = 2 marks |
1.7 Rewrite bar 1 of the right-hand part of the piano score in compound time. Add the new time signature.
Answer:
½ ½ 
Time signature = 1 mark Notation = 2 x ½ mark as indicated |
1.8 Write the scales below as indicated. Use semibreves.
1.8.1 Write F# melodic minor, ascending and descending, with key signature. Mark the semitones.
Answer: 
General assessment = 3 marks |
1.8.2 Write the Aeolian mode on E, descending in the alto clef.
Do NOT use a key signature.
Answer: 
Clef = 1 mark |
1.8.3 Write a chromatic scale on Bb, ascending only.
Answer:
(3)
General assessment = 3 marks |
[20]
QUESTION 2 (25 minutes)
Answer EITHER QUESTION 2.1 OR QUESTION 2.2.
2.1 Complete the opening motif below to form a twelve-bar melody in ternary form for any single-line melodic instrument of your choice. Name the instrument for which you are writing. Indicate the tempo and add dynamic and articulation marks.
Concept answer:
Instrument: Violin/Clarinet
Andante
OR
2.2
Concept answer:
Instrument: Cello/Trombone/Bassoon/Double Bass/Bass Guitar
Andante
The melody will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | ||
Form and cadential points | 1 mark per phrase x 3 | 3 | |
Correctness | Minus ½ mark per error up to 2 marks | 2 | |
Quality
| 9–10 | Excellent | 10 |
7–8 | Good | ||
4–6 | Average | ||
0–3 | Not acceptable | ||
TOTAL | Markers may use ½ marks | 15 | |
QUESTION 3 (10 minutes)
Answer EITHER QUESTION 3.1 OR QUESTION 3.2.
3.1 Study the extract by Mendelssohn below and answer the questions that follow.
Answer:
3.1.1 Name the key of this extract.
Answer:
- G major (1)
G major = 1 mark |
3.1.2 Identify the chords from (a)–(e) and figure them on the score. Use EITHER figuring below the score, for example V6, OR chord
symbols, for example C/E above the score.
Answer:
- See score (5)
1 mark per chord = 5 marks |
3.1.3 Name the types of non-chordal notes at (i) and (ii).
Answer:
- Passing note
- (Upper) Auxiliary (2)
(i) Passing note = 1 mark |
3.1.4 Which ONE of the following do you associate with the note at Z? Make a cross (X) in the appropriate block.
Anticipation | Suspension | Appoggiatura | Chord note |
Answer:(1)
Anticipation = 1 mark |
3.1.5 Name the cadence with which this extract ends.
Answer:
- Imperfect (cadence) (1)
Imperfect cadence = 1 mark (No marks if only chords are given) |
[10]
OR
3.2 Study the extract from Till by Charles Danvers below and answer the questions that follow.
Answer:
3.2.4 Imperfect cadence
3.2.1 Name the key of this extract.
Answer:
- F major (1)
F major = 1 mark |
3.2.2 Identify the chords from (a) to (e) and figure them on the score. Use chord symbols above the score, for example Bb/D
Answer:
- See score (5)
1 mark per chord = 5 marks |
3.2.3 Name the type of non-chordal notes at (i)–(iii).
Answer:
- (Chromatic lower) Auxiliary
- Suspension
- Anticipation (3)
(i) (Chromatic lower) Auxiliary = 1 mark |
3.2.4 Name the cadence at the end of this extract.
Answer:
- Imperfect (cadence) (1)
Imperfect = 1 mark |
[10]
QUESTION 4 (30 minutes)
Answer EITHER QUESTION 4.1 OR QUESTION 4.2.
4.1 Complete the four-part vocal harmonisation below by adding the alto, tenor and bass parts.
Concept answer:
The harmonisation will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS | |
Chord progression | 1 mark between each pair of chords (except between bar 5 and 6) | 14 | |
Correctness | Minus ½ mark per error but not more than 1 mark per chord | 16 | |
Quality |
| 10 | |
Note to marker: | 40 (÷ 8 x 3) | ||
TOTAL | 15 | ||
Candidates must be credited for an alternative and correct harmonisation not given in the memorandum. The figuring serves as a guide for the marker, but no marks are allocated for the symbols as such.
[15]
OR
4.2 Complete the piece below by adding a suitable bass line and harmonic material in the open staves. Continue in the style suggested by the given material in bars 1–4.
Concept answer:
The harmonisation will be marked according to the following criteria:
DESCRIPTION | MARK ALLOCATION | CANDIDATE'S MARKS | |
Chord progression | 1 mark between each pair of chords | 15 | |
Correctness | Minus ½ mark per error but not more than 1 mark per chord | 15 | |
Quality |
| 10 | |
Note to marker: | 40 (÷ 8 x 3) | ||
TOTAL | 15 | ||
Candidates must be credited for an alternative and correct harmonisation not given in the memorandum. The figuring serves as a guide for the marker, but no marks are allocated for the symbols as such.
TOTAL SECTION A: 60
SECTIONS B, C, D, E: GENERAL MUSIC KNOWLEDGE (90 minutes)
Answer SECTION B
AND SECTION C (Western Art Music)
OR SECTION D (Jazz)
OR SECTION E (Indigenous African Music).
Candidates must answer these questions in the ANSWER BOOK provided.
Note to the marker: One mark will be allocated for each correct fact. Candidates must be credited for any correct answer not given in this memorandum. |
SECTION B: GENERAL (COMPULSORY)
QUESTION 5
5.1
5.1.1 B
5.1.2 D
5.1.3 B
5.1.4 C
5.1.5 A
5.1.6 C
5.1.7 D
5.1.8 B
5.1.9 C
5.1.10 A (10)
1 mark for each correct answer = 10 marks |
5.2
5.2.1 Monophony
5.2.2 Homophony
5.2.3 Polyphony
5.2.4 Ostinato
5.2.5 Modes
5.2.6 Tone colour/Timbre
5.2.7 A Cappella
5.2.8 Mediant (5)
Any 5 correct answers = 5 marks |
If candidate answers more than 5 questions, mark ONLY THE FIRST 5 answers |
5.3
- Divided into two sections/AB
- Section A usually starts in the tonic key
- Modulates to the dominant (or to the relative major if tonic was a minor key)
- Section B starts in the new key and modulates back to the tonic
- Both sections contain repeat signs
- The music material is similar in both sections
- Phrases are usually symmetrical (5)
Any 5 correct facts = 5 marks |
TOTAL SECTION B: 20
Answer SECTION C (WAM)
OR SECTION D (JAZZ)
OR SECTION E (IAM).
SECTION C: WESTERN ART MUSIC (WAM)
QUESTION 6
Note to the marker: One mark will be allocated for each correct fact. Candidates must be credited for any correct answer not given in this memorandum. |
6.1
6.1.1 Pamina/Papagena
6.1.2 Tamino
6.1.3 Monostatos/Papageno
6.1.4 Sarastro
6.1.5 Queen of the Night (5)
Each correct answer = 1 mark |
6.2
- Has a German text throughout
- Includes a spoken dialogue
- Arias are often simply strophic or folk-like (Papageno's song)
- The plot contains
- Comical elements (Papageno)
- Romantic elements (Tamino and Pamina)
- Fairytale elements (Rescue of a Princess)
- Magical and fantasy elements (Magic flute/Magic bells)
- Characterisations of good and evil (Sarastro/Queen of the Night) (5) [10]
Any 5 correct facts = 5 marks |
QUESTION 7
7.1 Development (1)
Correct answer = 1 mark |
7.2 To facilitate the modulation from Theme 1 to Theme 2.
(Major tonic to dominant/Minor tonic to relative major) (1)
Correct answer = 1 mark |
7.3
- The music modulates to various keys
- Music material from the exposition is varied using different compositional devices
- New material may be included (2)
Any 2 correct answers = 2 marks |
7.4
- Both Themes of the Recapitulation are in the tonic key
- Exposition ends with codetta in dominant key and Recapitulation ends with Coda in tonic key (1) [5]
Any correct answer = 1 mark |
QUESTION 8
Title given by Beethoven to describe the programmatic content.
Hirtengesang. Frohe, dankbare Gefühle nach dem Sturm,
or
Shepherds' song. Feelings of joy and gratitude after the Storm,
or
Herderslied, Vrolike en dankbare gevoelens na die Storm
Any one answer = 1 mark |
Programmatic content realised through music:
- a portrayal of calmness, a general feeling of relief after the storm
- brings us back to the pastoral nature of the first movement
- pastoral mood created by yodelling figure/a shepherd's pipe is heard on clarinet; this is answered by another (horn), and then another call (violin)
- starts with a gentle, lyric theme followed by more vigorous sections
Tempo: Allegretto – reinforces pastoral association
Key: F major – tonic key of work helps to establish the return of pastoral mood
Harmony: Simple harmony/tonal stability to set a relaxed, easy-going atmosphere
Time signature: 6/8 – lilting, relaxed mood [5]
Any 4 correct facts = 4 marks |
QUESTION 9
Definition: orchestral work usually in four movements
Orchestral work = ½ mark |
Explanation of differences:
Classical Symphony | Beethoven: Pastoral Symphony |
Four movements | Five movements |
Standard Classical orchestra | Standard Classical orchestra with additional piccolo and trombone |
Modulation to relative keys | Modulation to relative keys and modulation to distantly related keys |
Absolute music | Programmatic |
No titles | Titles for each movement |
Classical forms: e.g. sonata form, sonata rondo | Fourth movement is in episodic/free form |
More limited range of tone colour | Expanded range of tone colour |
Coda not developed | Coda expanded Almost a new development |
Fourth movement: mostly in rondo or sonata or sonata-rondo form, in the tonic key and has a fast tempo | Fourth movement in episodical/free form |
[5]
Any 4 correctly correlated facts = 4 marks |
QUESTION 10
Classical Features
Form and structure:
- Uses Sonata form
Tone Colour/Instrumentation:
- Standard classical orchestra
Melodic Material:
- Motivic development
- Clearly defined melodies
Harmony:
- Modulations to relative keys
- Strongly influenced by Mozart and Beethoven
- Use of tonal/classical harmony
Texture:
- Mainly homophonic with some polyphonic texture
Any 6 correct facts = 6 marks |
Romantic Features
Form and structure:
- Uses Sonata form but more free and varied e.g. unusual long codetta after the second subject in the exposition
Programmatic:
- Use of descriptive title and subtitle
- Romanticism brought through a new focus on nature
- Suggests a whole seascape, the grandeur of the cave, the swelling of the sea, the light on the water, the fury of the waves breaking on the cliffs
- One of the first Romantic compositions to suggest nature in this way
New genre:
- Concert Overture (foreshadowing the Tone Poem to come)
Dynamics:
- Very wide range from pp to ff
- Tempo and Rhythm:
- Extensive use of rubato
Melody:
- Lyrical melodies with an individual melodic style (especially in the second subject)
Harmony:
- Some use of chromatic harmony
Any 6 correct facts = 6 marks |
CRITERIA | MARK ALLOCATION | ||
Classical features | 1 mark for each correct fact | 6 | |
Romantic features | 1 mark for each correct fact | 6 | |
Logical presentation and structure | Excellent | = 3 marks | 3 |
Good | = 2 marks | ||
Average | = 1½ marks | ||
Below average | = 1 mark | ||
Weak | = ½ mark | ||
Not acceptable | = 0 marks | ||
TOTAL | 15 | ||
[15]
TOTAL SECTION C: 40
OR
SECTION D: JAZZ
QUESTION 11
11.1
- Contrapuntal melodies and weaving of different melody lines together
- Melody developed through improvisation
- Solo pennywhistle (or saxophone) plays melody
- Moderate to upbeat tempo
- Skiffle-like beat
- Jive/Swing rhythms (3)
Any 3 correct facts = 3 marks |
11.2
- Electric guitar
- Bass guitar
- Drums
- Keyboard/Organ (3)
Any 3 correct answers = 3 marks |
11.3
11.3.1Meadowlands
11.3.2 Pata Pata or Lakutshon'ilanga
11.3.3 Sakhile or Isililo
11.3.4 Khoedi (The Moon) (4)
Any four correct answers = 4 marks |
[10]
QUESTION 12
12.1 | TRUE |
12.2 | TRUE |
12.3 | FALSE |
12.4 | FALSE |
12.5 | TRUE |
[5]
5 correct answers = 5 marks |
QUESTION 13
International:
- Combined and blended different styles
- Jazz
- Blues
- R&B
- Contemporary folk
- Gospel
- English ballads
- Portuguese fados
- Yiddish folk melodies
- Commercial popular music
- Recordings of American jazz artists, e.g. Ella Fitzgerald
Local:
- Traditional songs of the Xhosa and Zulu languages
- Explosive, clicking sounds of her mother tongue: isiXhosa
- Uses a cappella healing chants of the Amasangoma
Vocal quality:
- Rich, low female voice often compared in quality to Ella Fitzgerald's [5]
Any 2 correct international,2 local and 1 quality fact = 5 marks |
QUESTION 14
- South Africa's first important bebop band in the 1950s
- Band consisted of South African jazz-icons (Dollar Brand (later Abdullah Ibrahim) on piano, Kippie Moeketsi on alto saxophone, Jonas Gwangwa on trombone, Hugh Masekela on trumpet, Johnny Gertze on bass, Early Mabuza or Makaya Ntshoko on drums)
- Started a particularly South African sound which individual artists developed over the following decades
- 1959: First album by a black South African band, Jazz Epistle, Verse 1
- 1959: The musicians were involved in the popular South African jazz musical, King Kong which toured overseas
- Many of these musicians chose exile and developed their style further through contact with European musicians. They ploughed back their new-found knowledge and skills into the South African context on their return [5]
Any 5 correct facts = 5 marks |
QUESTION 15
Origins
- Started: 1920 in Johannesburg (Sophiatown)
- Ticky-draai (Cape folk dance)
- Xhosa folk songs
- Early American jazz
- Ragtime
- Blues music (12 bar blues)
- Music at parties of urban working class African musicians
- Used for social occasions, e.g. stokvel parties
- Music used at shebeens for entertainment
Any 5 facts for 5 marks |
Characteristics
- Primarily a keyboard style
- Other characteristic instruments include: simple pedal organ, guitar, banjo, drum (self-made), percussion, e.g. shakers
- Small instrumental ensemble
- Cyclic chord progression: I – IV ––164 ––V
- Rearranged traditional melodies in a three-chord pattern
- Moderate to upbeat tempo and rhythm
- Repetitive, single-themed dance tunes
Any 5 facts for 5 marks |
Any artist and song
- Jazz Maniacs: Gully Low Blues
- The Manhattan Brothers: Jikela Emaweni
- The Flying Jazz Queens: Langa More
Any 1 artist and 1 song = 2 marks |
The essay will be marked according to the following criteria:
CRITERIA | MARK ALLOCATION | ||
Origins | 1 mark for each correct fact | 5 | |
Characteristics | 1 mark for each correct fact | 5 | |
Artist and song | 1 mark for each correct fact | 2 | |
Logical presentation and structure of the essay | Excellent | = 3 marks | 3 |
Good | = 2 marks | ||
Average | = 1½ marks | ||
Below average | = 1 mark | ||
Weak | = ½ mark | ||
Not acceptable | = 0 marks | ||
TOTAL | 15 | ||
[15]
TOTAL SECTION D: 40
OR
SECTION E: INDIGENOUS AFRICAN MUSIC (IAM)
QUESTION 16
16.1
16.1.1 False - with Paul Simon (1)
16.1.2 False - Malombo/Philip Tabane/Malombo Jazzmen (1)
16.1.3 False - Mbaqanga (1)
16.1.4 False - Marabi (1)
16.2
- A style of music that fuses Bapedi and Vhavenda music with jazz
- African rhythms provided by the malombo and bongo drums
- An interplay between drums, guitar and flute (2)
Any 2 correct facts = 2 marks |
16.3
16.3.1
- Ikati: plectrum used for guitar playing
- Ikati produces a percussive sound typical of the style
- Ukuvamba refers to strumming of chords percussively with plectrum
16.3.2
- Ukupika: picking of guitar strings with fingers
- Amadoda (thumb) plays the lower strings
- Amantombazane (rest of the fingers) play the melody on the upper strings (4)
2 correct facts for each = 4 marks |
[10]
QUESTION 17
- A modern version/construct of the Bapedi music/dance kiba
- Uses modern instruments instead of traditional Bapedi instruments
- Uses any African language in addition to Sepedi
- Not exclusive to any cultural group
- Performed over a standard contemporary drumbeat style (e.g. disco, jazz)
- Merges drum melo-rhythms with pluro-vocal responses, crepitations and vocal lilting
- The following traditional drums are used: Ditinti; Moropa wa diatla/Moropa; Kiba [5]
Any 5 correct facts = 5 marks |
QUESTION 18
AmaZulu: Amahubo
Ceremonial features/functions:
- Channel communication between the ancestors and community
- Used to please the ancestors
- Serves to strengthen identity of the clan
- Only performed by elders of a clan
- Only wives who have passed menopause are allowed at the performance
- Performed at important rituals e.g. preparing for war
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Amahubo are clan anthems (sacred songs)
- Overlapping call and response is characteristic of the music
- Leading part is usually a solo voice and response is done by the chorus
- Listeners participate in the amahubo with emotional responses evoked by the performer
- Each clan has its own Ihubo
- Use of body percussion
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
[5]
OR
AmaSwati: Inkwahla
Ceremonial features/functions:
- First fruits celebration
- The Inkwahla songs are reserved only for the Inkwahla ceremony and the death of a king
- It is a sacred celebration that reinforces the king's role in the Swazi nation
- Core function is to spiritually cleanse the King and his nation before they eat newly harvested crops
- In the opening of the ceremony, the community chants the Inkwahla songs, standing in a formation of a crescent moon
- Lyrics of the songs are usually mournful and moving
- In the second phase, young unmarried men gather at the king's home to create an enclosure with branches from a sacred tree, Lusekwane
- During the closing ceremony, the community gathers in full moon formation and chants the Inkwahla with great solemnity
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- On the first day priests go into the sea, singing and dancing Inkwahla songs/dances, as they fill calabashes with water that will be used to cleanse the king
- On their return they sing the king's praises (Izibongo)
- On the second day young men sing a lullaby as they cut the Lusekwane branches to prepare for the king's re-birth
- During the ceremony warriors hold their shields and women hold thin long wands, waving them in time to the rhythm of the Inkwahla song
- Young unmarried men beat their shields against their sides in a steady rhythm likened to a mother rocking her baby
- The Umgubo, which is regarded as the national anthem, is performed to praise the king and end the day's activities
- Use of voice and body percussion, also includes shields and sticks
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
[5]
OR
AmaXhosa: Intonjane
Ceremonial features/functions:
- Seclusion period during female initiation
- One main song/dance is performed - Umngqungqo
- The Umngqungqo is performed by married women
- The song/dance is performed outdoors
- The women hold sticks in their right hands while dancing to imitate the men
- Umngqungqo music/dance lasts for 2 days
- Thereafter the men perform the Umdudo dance which lasts up to six days
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Two main dance movements, both characterised by walking, swaying and balancing on the ball of the foot then stomping the heel down are performed in a circle
- In the first movement they move clockwise, then change direction
- In the second movement they face inwards
- Dancers move with grace, shaking their shoulders
- Vocal parts are largely improvised
- There are no instruments, only the clapping of hands
- The Umdudo dance opens with the singing of Umyeyezelo and the beating of the Ingqongqo drum
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
[5]
OR
AmaNdebele: Luma
Ceremonial features/functions:
- First fruits celebration
- The community gathers at the king's home in February to celebrate the Ndebele heritage and welcome in the new year
- It is believed that the performance enables people to cast spells
- The performance of the song is strictly forbidden during any other time except the Luma celebration
- The Luma is believed to bring a sense of well-being in the nation
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Song/Dance used to rejoice and praise the king
- A special royal praise-song is performed (Luma song)
- The song is believed to bring rain
- The Luma song/dance is used to pray for fertility for crops
- Use of voice, dance and body percussion
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
[5]
OR
Basotho: Lebollo
Ceremonial features/functions:
- Seclusion period during male initiation
- Males learn cultural values through Likoma (songs)
- The Likoma are imparted by mentors – usually older initiated males
- In some clans each initiate has his own mentor to teach him Likoma
- The Likoma are secret (performed privately where only initiates are present)
- Likoma are chanted slowly in a low-pitched voice and accompanied by dance
- The leader of the initiates composes the songs
- Through the Likoma, the adult's identity is reinforced
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- While the initiates go through the pain of circumcision, they sing Mokorotlo songs to reinforce their masculinity
- Their cries are drowned by these songs
- When the initiation is complete, the boys sing Mangae songs in public to show pride in their new status as men
- The Mangaye songs are composed by the newly initiated men and give them the opportunity to express their thoughts and feelings
- At the graduation each initiate recites Dithoko (praise songs)
- This symbolises the boys entry into adulthood
- Use of voice and movement
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
[5]
OR
Bapedi: Byale
Ceremonial features/functions:
- Seclusion period during female initiation
- Before initiation a girl goes into seclusion for a week to undergo puberty rites
- At the end of the seclusion period the girl embarks on a period of preparation where she is taught initiation songs
- Musical instruments are used to embody spirits and to simulate animals and spirits
- Every morning the Byale form an S-shaped line and perform a song/dance with slow movement
- Mime is used to learn behaviour that is expected from the Byale as women and duties they will have to perform as adults
- The Byale create songs based on small models of animals they make to represent different human qualities
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Every night during the seclusion week, women gather in the initiates' hut for dancing, singing and drumming to prepare the girl for initiation
- Dances are characterised by movements representing the development of the child in the womb
- Songs characterised by singing in unison with rhythm created by plucking their lips with fingers
- The Byale's passage to a secluded spot in the mountain where the initiation will take place is marked by the eerie sound of a drum (Moshupiane) [5]
- The drum is played by an older woman, but the Byale are not allowed to see her or know the source of the sound
- The sound of the drum resembles a lion's roar
- It also symbolises the spirits of the mountain that will guard the Byale (initiates) during this period
- A drum (Moropa) is beaten throughout the night by older women during the initiation period to ensure strangers don't come near the initiation school
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
OR
Batswana: Bojale
Ceremonial features/functions:
- Seclusion period during female initiation
- Rite of passage among the Batswana people
- Introduces the initiates to basic values of their society
- The Bojale is done when girls reach puberty
- Girls are trained on what female duties entail
- Most initiation music has an educational function
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Bonwale (female initiates) participate in musical activities throughout the initiation period
- They have to memorise the secret formulae (Rupa) which they chant
- The main performance of initiation is the Radikgaratlane dance
- On the last night of initiation the Bonwale perform the Thojane dance
- When the girls return home they greet their families with songs (Mekgolokwane)
- A common concern with most female initiation songs is to discourage young girls from getting pregnant before marriage
- The Mekgolokwane songs are homecoming songs, but the dance could refer to the last days of pregnancy when a woman can't walk properly [5]
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
OR
Vhavenda: Domba
Ceremonial features/functions:
- Seclusion period during female initiation
- It is held throughout the year to accommodate the three phases of initiation – Vhusha, Tshikanda and Domba
- During the first two phases of Domba, singing and dancing take place mainly in the chief's huts
- The volume of the music is a reflection of a chief's prestige and wealth
- During Domba all instrumental music is banned in order to emphasize the sacredness of the ritual
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- In the first phase the initiates spend most of the time rehearsing the Ndayo dances o The learn by imitating the older girls
- There are special songs to mark rituals, such as the removal of each girl from their home
- During the final phase of Domba, the initiates learn the Tshikanda for a full month
- The most distinctive feature of the Domba is the python dance (Domba)
- The python symbolises fertility
- The initiates also learn the myth of Thovhela and Tshishongwe which they enact using music, dance and drama
- This teaches them moral ethics
- The Ngoma drum, reserved only for sacred rituals, is played during initiation [5]
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
OR
Batsonga: Mancomane
Ceremonial features/functions:
- Exorcism ritual
- Exorcism takes place nightly during summer
- The ritual takes its name from the Ncomane drum which is the defining feature of the exorcism
- Patients are individuals who have been possessed by unwanted Zulu or Ndau spirits
- The Dzwavi (diviner) possesses a set of four drums
- The drums are a symbol of the Dzwavi's authority
- They produce a sharp, powerful sound and are instrumental in inducing a hypnotic state in the patient.
Any 2 correct facts = 2 marks |
Role of dance, music and instruments:
- Three drum rhythms are used to expel different evil spirits: Mandlozi for Zulu spirits and Xidzimba and Xindau for Ndau spirits
- The drums are beaten with a stick (Rikhokho) very close to the patient's ear
- This is to create a disturbing sound for the spirits
- They are usually played with the Ngoma bass drum adopted from the Vhavenda
- Hand rattles (Njele) are also shaken and dancers wear leg-rattles (Marhonge)
- They are thought to be a source of the Batsonga ancestral spirits
- The songs used are in the language of the spirit being expelled
- Songs consist of repetition of short vocal refrains with melodies that have a persistent character
- Singing contributes to the hypnotic, trance-like state of the patient
- The audience can also go into a trance as a result of the singing [5]
Any 3 correct facts = 3 marks 2 + 3 = 5 marks |
QUESTION 19
- Used to affirm ancestry
- Used to relate stories about the clan
- Often interwoven into a song
- Usually at a fast tempo
- Usually dramatic and energetic delivery
- Often performed by mature men
- Used during different celebrations
- Chiefs/Kings have their own personal praise poet (5)
Any 5 correct facts = 5 marks |
[5]
QUESTION 20
Origins:
- Imbube choral singing is the basis of this style
- Fuses both African traditional singing and Western church hymns
- Originates from the Zulu word 'cothoza' – to walk on one's toes lightly
- Migrant workers established the style
- to entertain themselves
- to create a sense of community in their living areas
- Informal practices of migrant workers developed into performances
- Music performances at
- music competitions
- weddings and other celebrations
Any 3 facts for 3 marks |
Style characteristics:
- All-male choirs
- Usually a cappella
- Lyrics of songs in isiZulu
- Multi-layered vocal texture
- Often songs are in call and response, with the leader (Tenor 1) calling
- TTBB is used with one voice (leader) singing an extra tenor part
- Simple chord progressions sung in close harmony
- In the contemporary context Isicathamiya is fused with popular music ∙ Includes a smooth and quiet dance style with very subtle movements
Any 5 facts = 5 marks |
Contribution of Ladysmith Black Mambazo:
- Accessibility to a wider audience through:
- Collaboration with well-known artists (Paul Simon on his Graceland album)
- Recordings with the SABC and Gallo
- Winning many awards (e.g. Grammy Awards)
- Having appeared with Michael Jackson
- Popularising Isicathamiya internationally
- Use of English in the lyrics
- Joseph Shabalala emphasised accuracy of rhythm and pitch through quieter and lush harmonies in contrast to the louder styles of the 1950s
- Modified choreography to reflect the softer sound of tip-toeing through a performance rather than stomping loudly on the ground
- Founded The Ladysmith Black Mambazo Foundation with the aim to promote and teach children about the history of Isicathamiya music
- As a way of giving back to their community, Ladysmith Black Mambazo would hold numerous workshops on Isicathamiya:
- Resulting in a more polished tone in the upcoming groups
- The overall quality of performance improved
- Raised this art form to a new level
Any 4 facts = 4 marks |
The essay will be marked according to the following criteria:
CRITERIA | MARK ALLOCATION | ||
Origins | 1 mark for each correct fact | 3 | |
Style characteristics | 1 mark for each correct fact | 5 | |
Contribution of Ladysmith Black Mambazo | 1 mark for each correct fact | 4 | |
Logical presentation and structure of the essay | Excellent | = 3 marks | 3 |
Good | = 2 marks | ||
Average | = 1½ marks | ||
Below average | = 1 mark | ||
Weak | = ½ mark | ||
Not acceptable | = 0 marks | ||
TOTAL | 15 | ||
[15]
TOTAL SECTION E: 40
GRAND TOTAL: 120
MECHANICAL TECHNOLOGY GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
MECHANICAL TECHNOLOGY
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
MEMORANDUM
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
1.1 B ✔ (1)
1.2 D ✔ (1)
1.3 C ✔ (1)
1.4 C ✔ (1)
1.5 B ✔ (1)
1.6 D ✔ (1)
1.7 A ✔ (1)
1.8 B ✔ (1)
1.9 A ✔ (1)
1.10 B ✔ (1)
1.11 B ✔ (1)
1.12 B ✔ (1)
1.13 D ✔ (1)
1.14 D ✔ (1)
1.15 A ✔ (1)
1.16 C ✔ (1)
1.17 A ✔ (1)
1.18 B ✔ (1)
1.19 B ✔ (1)
1.20 A ✔ (1)
[20]
QUESTION 2: SAFETY
2.1 Safety – Coil spring compressor:
- Make certain that the diameter of the compressor bolts can take the pressure of the coil spring. ✔
- Do not exceed the maximum pressure. ✔
- Make sure the compressors are clean and free from oil. ✔
- Ensure that the compressors are in a good working condition. ✔
(Any 2 x 1) (2)
2.2 Safety – Hydraulic Press:
- Take notice of the predetermined pressure of the hydraulic press. ✔
- Ensure the pressure gauge is in a good working order. ✔
- Platform on which the work piece rests must be rigid and square with the cylinder of the press. ✔
- The prescribed equipment must be used. ✔
- Check for oil leaks. ✔
(Any 3 x 1) (3)
2.3 Safety – beam bender:
- Ensure the beam is clamped parallel to the backboard. ✔
- Do not leave plastic beams loaded for any length of time, they tend to creep. ✔
- All the weight must be gently dropped onto the hanger as to reduce inaccuracies due to friction. ✔
- Do not exceed the tester's maximum load. ✔
- Make sure the tester is stable. ✔
(Any 2 x 1) (2)
2.4 Testers:
2.4.1 Brinell Tester:
- The tester must be mounted rigidly on a worktable. ✔ (1)
2.4.2 Bearing and gear Puller:
- Make sure that the puller is at 90° to the work piece before you start to pull. ✔
- ∙ Ensure that the clamps are tight and will not slip from the work piece. ✔
(Any 1 x 1) (1)
2.4.3 Torsion tester:
- Get specification (torsion) of the different materials and the size of rods you would like to test. ✔ (1)
[10]
QUESTION 3: TOOLS AND EQUIPMENT
3.1 Fuel pressure:
- Faulty diaphragm ✔
- Clogged fuel filter ✔
- Faulty non return valves ✔
- Worn gasket ✔
(Any 2 x 1) (2)
3.2 Precision measuring instruments:
3.2.1 Depth micro-meter ✔
- Vernier calliper ✔
(Any 1 x 1) (1)
3.2.2 Screw-thread micro-meter ✔ (1)
3.3 Depth micro-meter reading:
- Reading = 50 + 1,5+ 0,49 ✔
= 51,99 mm. ✔ (2)
3.4 Multimeter measurements:
- DC current measurement ✔
- DC voltage measurement ✔
- AC measurement ✔
- Resistance measurement ✔
- Diode measurement ✔
- Continuity measurement ✔
(Any 2 x 1) (2)
3.5 Trace the cylinder leakage in an engine:
- Listen to at the carburettor for a hissing noise. ✔
- Listen at the exhaust pipe for a hissing noise. ✔
- Listen for hissing noise in the dipstick hole. ✔
- Listen to hissing noise by removing the filler cap on the tappet cover. ✔
- By checking whether there are bubbles in the radiator water for blown cylinder head gasket or cracked cylinder block. ✔
(Any 2 x 1) (2)
3.6 Uses of cooling pressure tester:
- To test if the pressure cap on the cooling system operates according to the prescribed pressure of the system. ✔
- To pump compressed air into the cooling system to determine whether they are any water leakage in the system. ✔ (2)
[12]
QUESTION 4: MATERIALS
4.1 Properties/characteristics:
4.1.1 Cementite:
- Hard and brittle ✔✔ (2)
4.1.2 Pearlite:
- Good ductility ✔
- Very hard ✔
- Strong and tough ✔
- Resistance to deformation ✔
(Any 2 x 1) (2)
4.2 Iron –carbon equilibrium diagram
4.2.1 Iron –carbon equilibrium diagram ✔ (1)
4.2.2
- – Ferrite + Pearlite ✔
- – Austenite + Ferrite ✔
- – Austenite ✔
- – Austenite + Cementite ✔
- – Ferrite + Cementite ✔ (5)
4.2.3 Austenite:
- Soft, ✔ grain structure fine ✔ (2)
4.3 720 °C ✔ (1)
[13]
QUESTION 5: TERMINOLOGY
5.1 Indexing:
- Indexing = 40 ✔
n
= 40 ÷ 2 ✔
118 2
= 20 ✔ (3)
59
No fullturnsand 20 holes in a 59 -hole plate
5.2 Milling processes:
- Up-cut milling (2)

- Downcut milling (2)

5.3 Calculate: Gib head key:
5.3.1
- Width = D
4
= 102 ✔
4
= 25,5 mm (2) ✔
5.3.2
- Thickness = D
6
= 102 ✔
6
= 17 mm (2) ✔
5.3.3
- Length = D × 1.5
= 102 × 1.5 ✔
= 153 mm (2) ✔
5.3.4
- Thickness at smallend(t ) T = − L
100
= 17 - 153 ✔
100
t = 17 - 1,53
= 15,47 mm (4) ✔
5.4 Calculate – Spur gear:
5.4.1
- Addendum = m
= 3 mm ✔ (1)
5.4.2
- Dedendum = 1,157m or = 1,25m
= 1,157 x 3 ✔ = 1,25 x 3 ✔
= 3,47 mm ✔ = 3,75 mm ✔ (2)
5.4.3
- Clearance = 0,157m or = 0,25m
= 0,157 x 3 ✔ = 0,25 x 3 ✔
= 0,47 mm ✔ = 0,75 mm ✔ (2)
5.4.4
- Module = PCD
T
PCD = m × T ✔
= 3 × 60
= 180mm ✔(2)
5.4.5
- OD = PCD + 2m
= 180 + 2(3)
= 180 + 6 ✔
= 186 mm ✔ (2)
5.4.6
- Cutting depth = 2,157 m or = 2,25 m
= 2,157 x 3 ✔ = 2,25 x 3 ✔
= 6,47 mm ✔ = 6,75 mm ✔ (2)
5.4.7
- Circular pitch = m x π
= 3 x π ✔
= 9,43 mm ✔ (2)
[30]
QUESTION 6: JOINING METHODS
6.1 Slag inclusion ✔ (1)
6.2 Visual inspection defects
- Shape of profile ✔
- Uniformity of surface ✔
- Overlap ✔
- Undercutting ✔
- Penetration bead ✔
- Root groove ✔
- Crack free ✔
(Any 4 x 1) (4)
6.3 Causes of incomplete penetration:
- Weld speed too fast ✔
- Joint design faulty ✔
- Electrode too large ✔
- Current too low ✔
(Any 2 x 1) (2)
6.4 Prevention of lack of fusion
- Adjust electrode size ✔
- Correct preparation of joint ✔
- Correct weld current ✔
- Correct arc length ✔
- Correct weld speed ✔
(Any 2 x 1) (2)
6.5 Destructive test
6.5.1 Machinability test ✔ (1)
6.5.2 Nick-break test ✔ (1)
6.5.3 Bend test ✔ (1)
6.6 Dye penetration test
- Clean the weld that needs to be tested. ✔
- Spray dye onto the surface and leave to penetrate. ✔✔
- Excess dye is cleaned away with a cleaning agent. ✔
- Allow surface to dry. ✔
- Spray a developer onto the surface to bring out the dye trapped in the crack. ✔
- The dye will show all the surface defects. ✔ (7)
6.7 Functions of MIG/MAGS components
6.7.1 Wire feed controller
- Feeds the consumable electrode wire to the welding gun at a constant predetermined speed. ✔✔ (2)
6.7.2 Welding gun
- Activates the supply of gas, power and wire feed✔✔ (2)
6.8 Purpose of inert gas
- The inert gas shields the molten pool from the atmospheric gases. ✔✔ (2)
[25]
QUESTION 7: FORCES
7.1 Forces 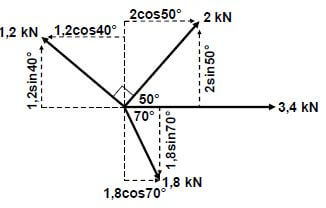
∑HC = 3,4 (√) + 1,8cos70º (√) - 1,2cos40º (√) + 2cos50º (√)
= 3,4 + 0,62 - 0,92 + 1,29
= 4,39 kN (√)
∑VC = 1,2 sin40 (√) + 2sin50º (√) - 1,8sin70º (√)
= 0,77 + 1,53 - 1,69
= 0,61 kN (√)
OR
Horizontal component | Magnitudes | Vertical component | Magnitudes |
-1,2cos40°✔ | -0,92 kN | 1,2sin40✔ | 0,77 |
3,4 ✔ | 3,4kN | 0 | 0 |
2cos50°✔ | 1,29kN | 2sin50°✔ | 1,53 |
1,8cos70°✔ | 0,62kN | -1,8sin70°✔ | 1,69 |
TOTAL | 4,39kN ✔ | TOTAL | 0,61kN ✔ |
- R2 = HC2 + VC2
R = √4,392 + 0.612
R = 4,43 kN - Tanθ = VC
HC
= 0,61
4,39
θ = 7,91º
R = 4,43 N at 7,91º northe of east (13)
7.2 Stress and Strain
7.2.1 Stress:
- A = π(D2 - d2)
4
A = π(0,0982 - 0,0672)
4
= 4,02 × 10-3 m2
σ = F
A
σ = 40000
4,02 × 10-3
σ = 9950248,76Pa
σ = 9,95 MPa (5)
7.2.2 Strain:
- ε = σ
E
ε = 9,95 × 106
90 × 109
= 0,11 × 10-3 or 1,11 × 10-4 (3)
7.2.3 Change in length
- ε = ΔI
oI
ΔI = ε × ol
= (0,11 × 10-3) × 0,08
=8,8 × 10-6m
= 8,8 × 10-3 mm (3)
7.3 Moments
Calculate A. Moments about B
- ∑RHM =∑ LHM
(A × 11,6) = (200 × 5,8) + (928 × 5,8) + (600 × 2,8)
11,6A = 1160 + 5382,4 + 1680
11,6A = 8222,4
11,6 11,6
A = 708,83 N
Calculate B. Moments about A
- ∑LHM =∑ RHM
(B × 11,6) = (600 × 8,8) + (928 × 5,8) + (200 × 5,8)
11,6B = 5280 + 5382,4 + 1160
11,6B = 11822,40
11,6 11,6
B = 1019,17 N (6)
[30]
QUESTION 8: MAINTENANCE
8.1 Preventative maintenance
- Can be described as maintenance of equipment or system before a fault occurs.✔ ✔ (2)
8.2 Lock out
- Locking out means that the machine's start switch cannot be activated without the knowledge of a servicing technician otherwise an accident would occur.✔ ✔ (2)
8.3 Clutch free-play
- The distance the pedal moves before the slack is taken from the linkage and release bearing. ✔ ✔ (2)
8.4 Viscosity index
- Viscosity index is a measure of how much the oil's viscosity changes as temperature changes. ✔ (1)
8.5 Replace clutch plate:
- Worn friction linings. ✔
- Weak or broken springs. ✔
- Glazed friction linings due to overheating. ✔
- Oil on friction linings. ✔
(Any 2 x 1) (2)
8.6 Grease – high viscosity
- To ensure that the grease coats and sticks ✔ to the bearing surfaces it is lubricating. ✔ (2)
8.7 Cutting fluid
- Mixture of soluble oil ✔ and water. ✔ (2)
8.8 Viscosity of cutting fluid
- Has a low viscosity to allow easy flow ✔ and effective dissipation of excess heat. ✔ (2)
[15]
QUESTION 9: SYSTEMS AND CONTROL
9.1 Gear drives
9.1.1 Rotation frequency of the output shaft
- NINPUT = TB × TD
NOUTPUT TA × TC
NOUTPUT = TA × TC × NINPUT
TB × TD
NIOTPUT = 18 × 16 × 1660
36 × 46
= 288,70 r/min (3)
9.2.2 Velocity Ratio
- VR = NINPUT
NOUTPUT
= 1660
288,70
= 5,75: 1 (2)
9.2 Belt Drives
9.2.1 Rotation frequency of the driver pulley π(D t) N
- V = π( D + t) × N
60
N = V × 60
π(D + t)
N = 36 × 60
π(230 + 12) × 10-3
2841,11r/min (4)
9.2.2 Power transmitted
- T1 = 2,5
T2
T1 = 2,5 × T2
=2,5 × 110
= 275 N - P = (T1 - T2)V
P = (275 - 110) × 36
= 5940W
5,94kW (4)
9.3 Hydraulics
9.3.1 Fluid pressure
- AB = πD2
4
= π × 0.0752
4
= 4.42 × 10-3 m2 - PB = F
AB
= 700 × 10 Pa
4.42 × 10-3
= 1583710, 41Pa
= 1583, 71 kPa ✔ (4)
9.3.2 Effort on piston A
- AA = πD2
= π × 0.042
4
= 1,256 × 10-3 m2 - PA = FA
AA
FA = PA × AA
=(1583,71 × 103) × (1,256 × 10-3)
= 1990,10 N
= 1,99 kN (4)
9.4 ABS
- Prevents wheel from locking during heavy breaking. ✔✔ (2)
9.5 Seat belt
- A seat belt has to be activated for its safety to be functional. ✔✔ (2)
[25]
QUESTION 10: TURBINES
10.1 Impulse Turbine
- Waterwheel ✔
- Pelton ✔
- Turgo ✔
- Michell – Banki/Crossflow/Ossberger✔
- Jonval turbine ✔
- Reverse overshot waterwheel ✔
- Archimedes' screw turbine ✔
(Any 2 x 1) (2)
10.2 Water turbine
10.2.1
- Water turbine ✔
- Kaplan-turbine ✔
- Reaction turbine ✔
(Any 1 x 1) (1)
10.2.2 Parts
- – Wicked gate ✔
- – Rotor ✔
- – Stator ✔
- – Shaft ✔
- – Water-flow ✔
- – Blades ✔ (6)
10.2.3 Advantages of water turbine
- Low maintenance ✔
- No need for lubrication ✔
- Fewer moving parts ✔
- Environmental friendly ✔
- Cost effective ✔
(Any 2 x 1) (2)
10.3 Turbines
10.3.1 Advantage of supercharger:
- Increases the output power of the engine. ✔
- A smaller engine fitted with a centrifugal blower delivers the same power as a larger engine. ✔
- It eliminates lack of oxygen above sea level. ✔
- Increases the volumetric efficiency of the engine. ✔
- With the aid of the intercooler both the power and the torque output of the engine are increased. ✔
(Any 2 x 1) (2)
10.3.2 Advantages of steam turbines:
- It is compact. ✔
- No lubrication is required. ✔
- Steam turbine speeds can be more accurately regulated. ✔
- A variety of fuels can be used to obtain steam. ✔
- Steam turbines are more economical. ✔
- Higher speeds can be obtained as compared to internal combustion engine. ✔
- Convert heat energy into mechanical energy. ✔
(Any 2 x 1) (2)
10.3.3 Advantages of gas turbines:
- Very high power to weight ratio ✔
- Smaller than most reciprocating engines of the same power rate ✔
- Moves in one direction only, with far less vibration ✔
- Low operating pressures ✔
- High operating speeds ✔
- Low lubricating oil cost and consumption ✔
(Any 2 x 1) (2)
10.4 Turbo lag
- It is a delay ✔ between pushing on the accelerator ✔ and feeling turbo kick in. ✔ (3)
[20]
TOTAL: 200