Adele
Religion Studies Paper 1 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
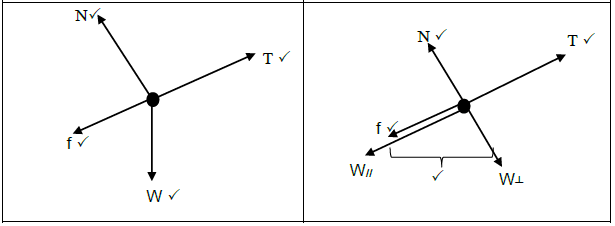
INSTRUCTIONS AND INFORMATION
- The question paper consists of SECTION A and SECTION B.
- SECTION A: COMPULSARY
SECTION B: Answer ANY TWO questions in this section. - The questions in SECTION A count ONE mark per fact, unless otherwise indicated.
- Read all the questions carefully.
- The length of your answers must be in accordance with the marks allocated to each question.
- Number the answers correctly according to the numbering system used in the question paper.
- Write neatly and legibly.
QUESTIONS
SECTION A (COMPULSORY)
QUESTION 1
1.1 Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question number (1.1.1–1.1.10) in the ANSWER BOOK, for example
1.1.11 D.
1.1.1 Orthodox Jews observe religious laws which are called …
- Sharia.
- Mitzvot.
- Genesis.
- Halakhah. (1)
1.1.2 The Tao may be described as the …
- path of the universe.
- earth.
- hereafter.
- cycle of life. (1)
1.1.3 The highest caste in traditional Hinduism is the …
- Upanishads or scribes.
- Sudras or labourers.
- Ksatriyas or rulers and warriors.
- Brahamanas or spiritual teachers. (1)
1.1.4 The founder of Buddhism is …
- Siddhartha Gautama.
- Baha’u’llah.
- Vajrayana.
- the Dalai Lama. (1)
1.1.5 The Baha’i faith originated in …
- Iraq.
- Iran.
- China.
- Saudi Arabia. (1)
1.1.6 When Prophet Muhammad passed away, he was succeeded by …
- Isaac.
- Abu Bakr.
- Jesus.
- Ali. (1)
1.1.7 An example of Neo- (modern) Hinduism is …
- Rig Veda.
- the Divine Life Society.
- the Bhagavad Gita.
- Bhakti yoga. (1)
1.1.8 The Hadith is/are ….
- a collection of teachings of the Prophet Muhammad.
- compulsory teachings in the Qur’an.
- books of myths.
- records of Islamic history. (1)
1.1.9 The oldest form of Buddhism is …
- Zen Buddhism.
- Tibetan Buddhism.
- Mahanyana Buddhism.
- Theravada Buddhism. (1)
1.1.10 A dogma, in a religious context, is …
- a lie or fabrication.
- teachings with absolute authority.
- a sacred text.
- a discussion of spiritual truths through a story. (1)
1.2 Choose an item from COLUMN B that matches an item in COLUMN A. Write only the letter (A–H) next to the question number (1.2.1–1.2.6) in the ANSWER BOOK, for example 1.2.7 I. Do NOT use any letter more than ONCE.
COLUMN A | COLUMN B | ||
1.2.1 | Following the model of the Holy Trinity, humanity should have a relationship of mutual respect and love | A | Traditional Hinduism |
1.2.2 | A sacred text consisting of a collection of laws and teachings | B | Torah |
1.2.3 | While there are several paths to God, the proper performance of domestic and temple rituals is obligatory for all | C | Islam |
1.2.4 | Religious stories in which deep truths about life are revealed | D | Kitáb-i-Aqdas |
1.2.5 | Two religious groups parted ways because of political differences | E | Christianity |
1.2.6 | A holy religious occasion | F | Myth |
G | Qur’an | ||
H | Ritual | ||
(6 x 1) (6)
1.3 Choose the word in EACH list below that does NOT match the rest. Write down the word next to the question numbers (1.3.1–1.3.5) in the ANSWER BOOK and give a reason why it does NOT fit.
EXAMPLE: Banana; Apple; Potato; Grape ANSWER: 1.3.6 Potato. The others are all fruit.
1.3.1 Charles Darwin; Shogi Effendi; Copernicus; Kepler (2)
1.3.2 Therevada; Pali Canon; Sanskrit; Mahayana (2)
1.3.3 Karma; Ahimsa; Tripitaka; Dharma (2)
1.3.4 Taoism; Buddhism; African Traditional Religion; Judaism (2)
1.3.5 Brahmo Samaj; Ramakrishna; Arya Samaj; Ecumenism (2)
1.4 Explain EACH of the following concepts in the context of religion:
1.4.1 Moksha (2)
1.4.2 Nation state (2)
1.4.3 Non-theistic (2)
1.4.4 Inspiration (2)
1.4.5 Atheism (2)
1.5 Indicate whether the following statements are TRUE or FALSE. Write ‘true’ or ‘false’ next to the question numbers (1.5.1–1.5.5) in the ANSWER BOOK. Correct the statement if it is FALSE.
1.5.1 Syncretism is the science of the interpretation of texts. (2)
1.5.2 A shaman is a Supreme Being; the Creator in African Traditional Religion. (2)
1.5.3 Bukhari is a collection of Marxist teachings. (2)
1.5.4 The New Testament is the sacred text of Judaism. (2)
1.6 Answer the following questions.
1.6.1 What is a normative source? (2)
1.6.2 Briefly explain the concept secularism. (4)
TOTAL SECTION A: 50
SECTION B
Answer ANY TWO questions in this section.
QUESTION 2
Read the extract below and answer the questions that follow.
UNIQUE (AND SOMETIMES DANGEROUS) RELIGIOUS RITUALS AROUND THE WORLD Throughout the world, believers adhere to practices that strike outsiders as bizarre, but seem completely reasonable to the faithful. [Extract taken from bootsnall.com. Accessed on 05 April 2020.] |
2.1 Write notes on the term UNIQUENESS in order to highlight religious uniqueness. (4)
2.2 Mention only ONE factor from the extract that forms religious identity. (2)
2.3 Hindus and Westerners believe in the tossing of an infant. Write down the FUNCTIONS of the uniqueness of a religion. (6)
2.4 Explain THREE unique features of any ONE religion in EACH of the groupings below:
2.4.1 Eastern religions (6)
2.4.2 Middle Eastern religions (6)
2.5 Name FOUR similarities that exist within the Abrahamic religions. (8)
2.6 In the context of religion, give TWO facts about EACH of the following:
2.6.1 Hinduism’s view on Karma (4)
2.6.2 The Inyanga in African Traditional Religion (4)
2.6.3 Canon in the Christian religion (4)
2.7 Briefly discuss EACH of the following concepts in the context of religion:
2.7.1 Similarities (2)
2.7.2 Identity (2)
2.7.3 Differences (2)
[50]
QUESTION 3
Read the extract below and answer the questions that follow.
IN SOUTH AFRICA, THE WORLD’S RELIGIOUS LEADERS GATHER FOR DIALOGUE AND ACTION Cape Town, South Africa – Without doubt, one of the highlights for religious leaders gathered here for the Parliament of the World’s Religions was a speech by a secular political leader: former South African President Nelson Mandela. Addressing the thousands of representatives gathered from the world’s major faith groups, the 81-year-old former political prisoner said the religious institutions played a major role in bringing about the end of apartheid in South Africa. ‘Without the Church and religious institutions, I would never be here today’, said President Mandela, explaining that it was Christian, Muslim, Hindu and Jewish religious groups that were instrumental in providing him and other young blacks with an education – and later in giving comfort to political prisoners and their families. President Mandela went on to say that, “Religion will have a crucial role to play in guiding and inspiring humanity to meet the enormous challenges we face in the next century”. [Extract taken from onecountry.org. Accessed on 05 April 2020.] |
3.1.1 Define interreligious dialogue. (2)
3.1.2 Give a synonym for the word ‘instrumental’ in paragraph 3. (2)
3.1.3 Four different religions are mentioned in the extract. Explain briefly how these religions impacted President Mandela. (6)
3.1.4 President Mandela asked religious leaders to work together. Name and discuss an organisation that promotes inter-religious dialogue in South Africa. (8)
3.1.5 Discuss interreligious dialogue in South Africa prior to 1996. (10)
3.1.6 Do you think women are playing an important role in promoting interreligious relationships in South Africa? Give reasons for your answer. (8)
3.2 Read the following extracts and answer the questions that follow.
CHRISTIAN RESPONSE NEEDED AGAINST THREATS TO REMOVE CHRISTMAS AND GOOD FRIDAY HOLIDAYS The recent proposal by the South African Law Reform Commission (SALRC) to remove Good Friday and Christmas as public holidays on the SA calendar, has, triggered criticism around the country. [Extract taken from gatewaynews.co.za. Accessed on 05 April 2020.] |
DEBATES CONTINUE OVER RELIGIOUS HOLIDAYS Durban – African Christian Democratic Party president Reverend Kenneth Meshoe has told Christians to reject any attempts by the government to scrap Good Friday and Christmas as public holidays on the South African calendar. [Extract taken from iol.co.za. Accessed on 15 December 2019.] |
3.2.1 What are the reasons given by the SALRC for removing Christmas and Good Friday from the SA calendar? (4)
3.2.2 What reasonable arguments can Christians put forward for keeping the holidays on the calendar? (4)
3.2.3 According to the writer, what alternatives exist according to the Public Holidays Act for adherents of other religions? (4)
3.2.4 It is hard to accept that these holidays serve to ‘deepen religious divides.’ What do you think this quotation mean? (2)
[50]
QUESTION 4
4.1 Read the following extract and answer the questions that follow.
“Allah is the Light of the heavens and the earth. The parable of His light is as if it were a niche containing a lamp; the lamp is [enclosed] in glass, the glass [shining] like a radiant star: [a lamp] lit from a blessed tree – an olive-tree that is neither of the east nor of the west, the oil whereof [is so bright that it] would be well-nigh give light [of itself] even though fire had not touched it: light upon light! Allah guides unto His light him that wills [to be guided]; and [to this end] Allah propounds parables unto men, since Allah [alone] has full knowledge of all things.”“Allah is the Light of the heavens and the earth. The parable of His light is as if it were a niche containing a lamp; the lamp is [enclosed] in glass, the glass [shining] like a radiant star: [a lamp] lit from a blessed tree – an olive-tree that is neither of the east nor of the west, the oil whereof [is so bright that it] would be well-nigh give light [of itself] even though fire had not touched it: light upon light! Allah guides unto His light him that wills [to be guided]; and [to this end] Allah propounds parables unto men, since Allah [alone] has full knowledge of all things.” [Source: The Quran 24:35] |
4.1.1 In the context of religion explain the concept parable. (4)
4.1.2 Give an opinion on what the lesson or spiritual truth of ‘Allah is the Light’ (the parable) is. (4)
4.1.3 Name the elements described in the parable. (4)
4.1.4 Name a parable (except ‘Allah is the Light’) from any religion and explain its meaning. (8)
4.2 ‘Doctrines are the beliefs that provide the central frame of reference for a religion.’ Give ONE example of a doctrine from EACH of the following religions and explain its meaning:
4.2.1 Christianity (4)
4.2.2 Hinduism (4)
4.3 In your own words explain ONE well-known dogma of the Roman Catholic church. Write short notes. (6)
4.4 Explain the following terms in the context of religion:
4.4.1 Belief (8)
4.4.2 Ideology (8)
[50]
QUESTION 5
5.1 Read the following extract and answer the questions that follow.
SOUTH AFRICA DECLARES ‘FEMICIDE’ A NATIONAL CRISIS The South African government has declared gender-based violence a national crisis. According to a new government report, a woman is murdered every three hours in South Africa, and many are assaulted and raped before their death. [Extract taken from www.voanews.com˃africa. Accessed on 08 January 2020.] |
PROTESTERS OUTSIDE PARLIAMENT DEMAND ACTION ON FEMICIDE, NOT JUST WORDS Ongoing protests against femicide and gender-based violence have forced President Cyril Ramaphosa’s government to relook laws on how convicted abusers, rapists, and murderers should be punished. [Extract taken from mg.co.za. Accessed on 21 November 2019.] |
5.1.1 Explain TWO women’s or girls’ rights that are being violated through gender-based violence. (4)
5.1.2 The South African constitution protects human rights. Explain what is meant by this statement. (4)
5.1.3 Do you think the steps that President Ramaphosa suggested can be effective to stem the tide of gender-based violence? Give reasons for your answer. (6)
5.1.4 What campaigns can religious organisations employ to address the problem of gender-based violence? (6)
5.1.5 In South Africa, a woman is murdered every three hours. What measures do you think can the government introduce to stop femicide? (6)
5.1.6 From the extract, give evidence that perpetrators of gender-based violence often treat women and children in an inhumane manner. (4)
5.1.7 Mention any TWO incidents of femicide in South Africa that you are aware off. (4)
5.2 Read the following extract and answer the questions that follow.
16 DAYS OF ACTIVISM FOR NO VIOLENCE AGAINST WOMEN AND CHILDREN It is a worldwide campaign to oppose violence against women and children. Its aim is to raise awareness of the NEGATIVE impact that violence and abuse have on women and children and to rid society of abuse permanently. [Extract taken from www.parliament.gov.za. Accessed on 15 December 2019.] |
5.2.1 Give a synonym for activism. (2)
5.2.2 According to the extract, what are the aims of the 16 Days of Activism campaign? (4)
5.2.3 Quote ONE word from paragraph 1 (ONE) that tells us that this is not only a South African initiative. (2)
5.2.4 What do you think are possible causes of gender-based violence? (4)
5.2.5 Do you think the desired aim can only be accomplished between 25 November and 10 December? Motivate your answer. (4)
[50]
TOTAL SECTION B: 100
GRAND TOTAL: 150
Technical Sciences Paper 2 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
MEMORANDUM
QUESTION 1
1.1 B (2)
1.2 A (2)
1.3 C (2)
1.4 A (2)
1.5 C (2)
1.6 C (2)
1.7 D (2)
1.8 D (2)
1.9 A (2)
1.10 B (2)
[20]
QUESTION 2
2.1 A series of organic molecules that can be described by the same general formula and where each member differs from the next by a CH2 group. (2)
2.2 A group of atoms whose bonding is the same from molecule to molecule with similar physical and chemical properties. (2)
2.3
2.3.1 E (1)
2.3.2 C (1)
2.4
2.4.1 Butane/Butaan (2)
2.4.2 2-methylpropan-1-ol/ 2-metielpropan-1-ol OR 2-methylpropanol/2-metielpropanol (2)
2.5
2.5.1 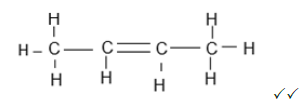 (2)
(2)
2.5.2 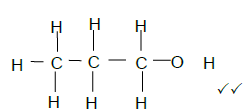 (2)
(2)
2.5.3 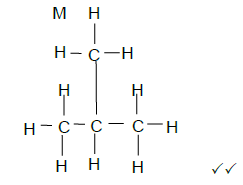 (2)
(2)
2.6 Positional isomers are organic molecules with the same molecular formula/ and same structural formula/ and same functional group, but differ from each other in the location (position) of the functional group in the carbon chain. (2)
2.7 Ketones/Ketoon (1)
2.8 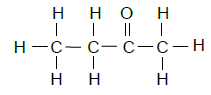 (2)
(2)
[21]
QUESTION 3
3.1 It is a long chain of monomers, covalently bonded together (in a repeating patterns). (2)
3.2 Unsaturated Not all C-C bonds are single bonds
OR
It contains C-C double bonds (3)
3.3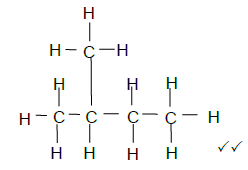 (2)
(2)
3.4
- Destruction of indigenous forests (leading to global warming)
- Rubber is not biodegradable – disposal impacts negatively on environment
- Burning of rubber releases toxic gases (Any 2) (2)
3.5
- Job creation
- Tyres for cars / gloves for medical industry / raincoats etc
- Protective devices – insulation (Any 2 x 2) (4)
[13]
QUESTION 4
4.1 D (2)
4.2
4.2.1 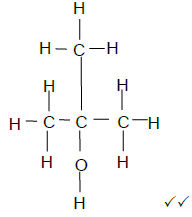 (2)
(2)
4.2.2 D (2)
4.3
4.3.1 C (propan-1-ol/1-propanol)
Longest flow time / flows the slowest / most resistance to flow. (2)
4.3.2
- Increase in chain length / molecular mass / molecular size / surface area from A to C.
- Increase in strength of intermolecular/ Van der Waals / Dispersion / London forces. (3)
4.3.3 C. Since it is having strong intermolecular forces and the longest chain length which contributes to its resistance to flow makes propan-1-ol a best lubricant. (3)
4.3.4 Vapour pressure is a measure of the tendency of a material to change into a gaseous state. (2)
4.4
- E (butan-2-ol or 2-butanol)
The more branched/more compact alcohol/E has a smaller surface area (over which the intermolecular forces act).
Decrease in strength of intermolecular forces/
reduced resistance to flow (and thus lower viscosity).
OR - The straight-chain alcohol/D has a larger surface area/less compact (over which intermolecular forces act).
Increase in strength in intermolecular forces.
Increased resistance to flow (and thus higher viscosity). (3)
[19]
QUESTION 5
5.1 Addition (reaction) (2)
5.2
- Add acidic reagent HBr
- At room temperatures OR 25ºC (2)
5.3 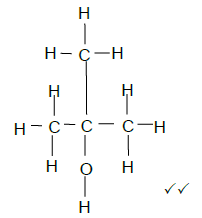 (2)
(2)
5.4 H2O OR Water (2)
5.5 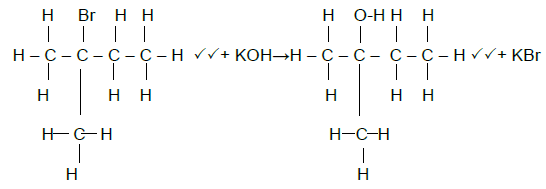 (4)
(4)
5.6 Addition (reaction) (2)
5.7
5.7.1 Water and Carbon Dioxide (2)
5.7.2 2 C6H14 + 19 O2 → 12 CO2 + 14 H2O
( balancing is 1 mark) (4)
[20]
QUESTION 6
6.1 Ethanol (2)
6.2 It acts as a catalyst(2)
6.3 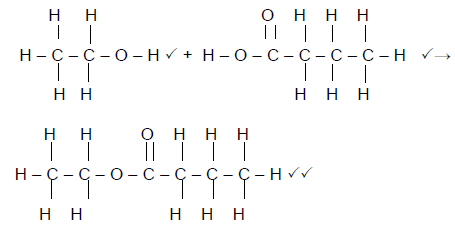 (4)
(4)
6.4 The contents of the mixture are flammable (2)
6.5 They are used to flavour foods and sweets (2)
7.1
- Transverse
- Waves are carried by the medium
OR
Waves travel through a medium - Wavelength (λ) per the period (T) of a wave (6)
7.2
7.2.1 Incident ray (1)
7.2.2 Refracted ray (1)
7.2.3 Normal (1)
7.2.4 Refracted angle(1)
7.3 Refraction is the bending of light when it passes from one incident ray from one optical medium to another. (2)
[12]
QUESTION 8
8.1 Total internal reflection/Totale interne weerkaatsing (1)
8.2 It is when the incident ray completely reflects back to the optical denser medium. (2)
8.3
- Light must travel from a denser optical medium to a less dense optical medium.
- The incident angle must be greater than the critical angle (2)
8.4
- In medicine
- In submarines
- In telecommunications
- In cameras (Any 2) (2)
8.5 It is an angle of incidence in the denser medium such that the refracted ray just passes through the surface of separation of the two mediums. (2)
8.6 The incident ray, the reflected ray and the normal to the surface all lie in the same plane and the angle of reflection Өr equals the angle of incidence Өi. (2)
8.7
8.7.1 Dispersion (1)
8.7.2 It is the spreading of the white light when entering an optical denser medium into its primary colours. (2)
8.8
8.8.1 Convex OR Converging(1)
8.8.2 It is the distance between the centre of a lens and its focus. (2)
8.8.3
- Virtual image
- Upright
- Enlarged (3)
[20]
QUESTION 9
9.1
- Radio waves
- Microwaves
- Infrared
- Visible light
- Ultraviolet rays
- X-rays
- Gamma rays (2)
9.2 Gamma rays (1)
9.3 It has the highest frequency; according to the formula E = hf, the higher the frequency, the higher the energy of a photon. (2)
9.4 It is a wave with a changing magnetic and electric field perpendicular to each other in the direction of propagation of the wave. (2)
9.5 Option 1
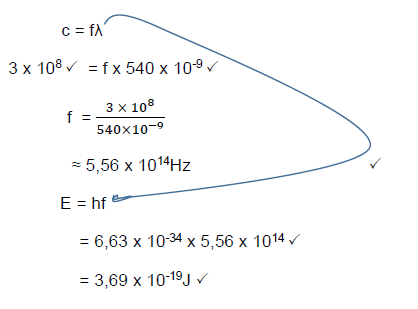
Option 2
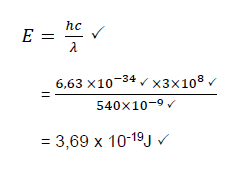 (5)
(5)
9.6 GREATER THAN (1)
[13]
TOTAL: 150
Technical Sciences Paper 1 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
MEMORANDUM
QUESTION 1
1.1 C (2)
1.2 D (2)
1.3 C (2)
1.4 A (2)
1.5 C (2)
1.6 A (2)
1.7 D (2)
1.8 D (2)
1.9 B (2)
1.10 A (2)
[20]
QUESTION 2
2.1
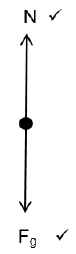 (2)
(2)
2.2 An object will continue its state of rest or uniform motion in a straight line unless it is acted upon by a net force. (2)
2.3 Block will continue its state of uniform motion (constant velocity). (2)
[6]
QUESTION 3
3.1 When a net force acts on an object, the object will accelerate in the direction of the force. This acceleration is directly proportional to the net force and inversely proportional to the mass of the object. (2)
3.2 No net external force act on the system. (2)
3.3
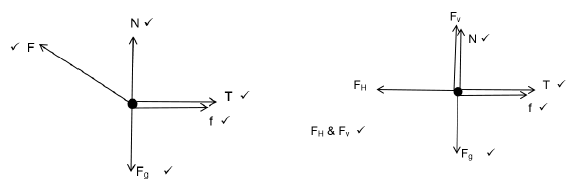 (5)
(5)
3.4 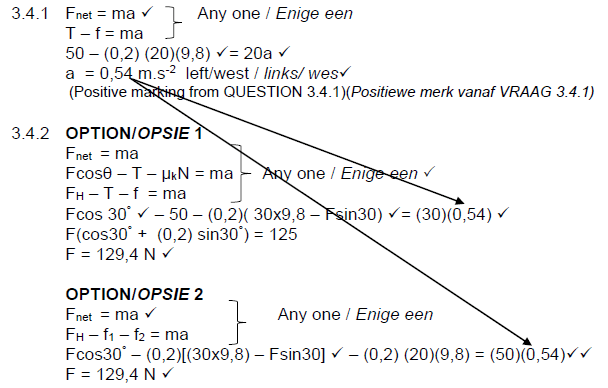
3.5 3.5.1 Decreases (1)
3.5.2 Decreases (1)
3.6 Fnet decreases, therefore acceleration decreases. (2)
[22]
QUESTION 4
4.1 Momentum is the product of mass of the object and its velocity. (2)
4.2 Momentum (1)
4.3
- ∑pi = ∑pf East = +ve)(Oos = +) Any ONE
m1v1i + m2v2i = m1v1f + m2v2f
(5)(4) + (3)(0) = (5)( - 0,25) + (3)(v2f)
v2f = 7,08 m.s-1 east (4)
4.4
- Law of conservation of momentum
(Negative marking)
Total linear momentum in a closed system remains constant. (3)
[10]
QUESTION 5
5.1 100 000 N due east (2)
5.2 -ve Newton’s Third law of Motion - When object A exerts a force on object B, object B simultaneously exerts an oppositely directed force of equal magnitude on object A. (3)
5.3 It is the product of the resultant/net force acting on an object and the time the resultant/net force acts on the object. (2)
5.4
- OPTION 1
West is taken as positive
Fnet ∆t = ∆p
Fnet(truck) ∆t = ( mvf – mvi)
(- 100000)(0,4) = (5000)[(vf) – 20]
vf = 12 m.s-1 west - OPTION 2
East is taken as positive
Fnet ∆t = ∆p
Fnet(truck) ∆t = ( mvf – mvi)
(100000)(0,4) = (5 000)[(vf) – (-20)]
vf = - 12 m.s-1
vf = 12 m.s-1 west (4)
[11]
QUESTION 6
6.1 6.1.1 Work done is defined as the product of the force applied on an object and the displacement in the direction of the force. (2)
6.1.2 Tension Force of gravity (2)
6.1.3 Fnet = 0
Block of bricks is moving with constant velocity (2)
6.1.4
- P = Fv F = mg = (50)(9,8) = 490 N
P = (490)(5)
P = 2 450 W (4)
6.2
6.2.1 Total mechanical energy of a closed system remains constant. (2)
6.2.2
- ME at a height h above the ground:
MEh = ½mv2 + mgh
MEh = ½(0,5)(10)2 + (0,5)(9,8)h …….. (1)
ME⅔h = ½(0,5)(20,34)2 + (0,5)(9,8)(⅔h ) …… (2)
(1) = (2)
½(0,5)(10)2 + (0,5)(9,8)h = ½(0,5)(20,34)2 + (0,5)(9,8)(⅔h )
(⅓h)(9,8)(0,5) = 78,43
h = 48,02 m
ME = ½(0,5)(10)2 + (0,5)(9,8)(48,02)
ME = 260,3 J (6)
6.2.3
- (ME)at h = (ME)ground ?
260,3 = mgh + ½mv2
260,3 = 0 + ½(0,5)(v2) ?
v = 32,27 m.s-1 ? (3)
6.3
6.3.1 0 J ? Normal force is perpendicular to the direction of the displacement. (3)
6.3.2
- W = F∆xcosθ ?
W = (10)(4)(cos0°) ?
W = 40 J ? (3)
[27]
QUESTION 7
7.1 A body which regains its original shape and size completely when the deforming force is removed. (2)
7.2 The internal restoring force per unit area is called stress. (2)
7.3 7.3.1 Stress is directly proportional to strain. (2)
7.3.2 Hooke’s law (1)
7.3.3 Tungsten
Graph of tungsten is steeper than steel. (2)
7.3.4
- m = 5,4 ×109−0
3×10−2−0
m = 1,8 × 1011
Modulus of elasticity = 1,8 × 1011 Pa (3)
7.4
7.4.1 The property of the fluid to oppose relative motion between the two adjacent layers. (2)
7.4.2 Water
The lower the viscosity, the faster the flow. (2)
7.4.3 As the temperature of the fluid increases, its viscosity decreases. (2)
7.5
7.5.1 Pascal's law states that in a continuous liquid at equilibrium, the pressure applied at a point is transmitted equally to the other parts of the liquid. (2)
7.5.2
- F1 = F2 ?
A1 A2
1765 ?= F2 ?
284×10−6 507×10−6
F2 = 3 150,9 N (4)
7.5.3 Increases (1)
7.5.4 Bulldozer’s working systems, dentists’ chairs, hydraulic lifts used to lift heavy loads, car jacks (ANY TWO) (2)
[27]
QUESTION 8
8.1
8.1.1 The process of adding impurities to intrinsic / inpure semiconductors. (2)
8.1.2 5 (1)
8.1.3 n-type (2)
8.1.4 In n-type material current is due to electrons whereas in p-type the current is due to holes. (2)
8.2
8.2.1
- C = ?0?
?
C = 8,85 ×10−12×10×10−4
1×10-2
C = 8,85 × 10-13 F
Q = CV
Q= (8,85 × 10-13)(12)
Q = 1,06 × 10-11 C (6)
8.2.2 Zero (1)
8.2.3
- Area of the plate
- Distance between the plate
- Dielectric used ( Any TWO) (2)
8.3
8.3.1 Energy transferred per second. (2)
8.3.2
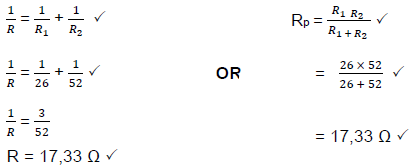 (3)
(3)
8.3.3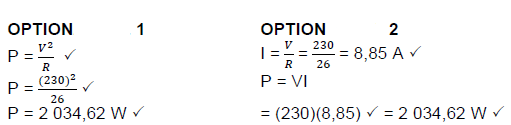 (3)
(3)
8.3.4 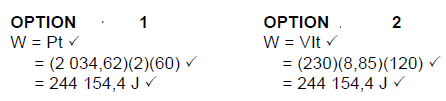 (3)
(3)
[27]
TOTAL: 150
Technical Sciences Paper 2 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
Read the following instructions carefully before answering the questions.
- Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- You may use a non-programmable calculator.
- Leave ONE line between two sub-questions for example between QUESTION 2.1 and QUESTION 2.2.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers
(1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 Which ONE of the following organic compounds does NOT contain a carbonyl group?
- Aldehydes
- Alcohols
- Ketones
- Esters (2)
1.2 Which ONE of the following general formulae represents alkynes?
- CnH2n-2
- CnH2n-1
- CnH2n
- CnH2n+2 (2)
1.3 Which ONE of the following pairs of reactants can be used to prepare the ester ethyl butanoate in the laboratory?
- Ethanal and butanol
- Ethanoic acid and butanol
- Ethanol and butanoic acid
- Ethanal and butanoic acid (2)
1.4 Which ONE of the following compounds represents a ketone?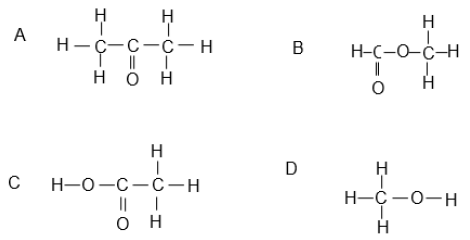
(2)
1.5 The structural formula of a haloalkane is represented below.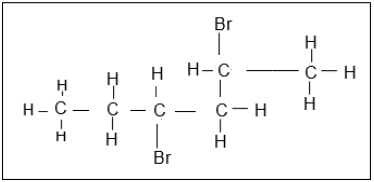
Which ONE of the following descriptions is the correct IUPAC NAME for this organic molecule?
- 3,5-dibromohexane
- 4-bromo-5-bromo-5-methylpentane
- 2,4-dibromohexane
- 2-bromo-1-bromo-1-methylpentane (2)
1.6 A ray of light strikes a glass block at an angle θ, as shown below. The ray passes through the glass block and emerges on the opposite side.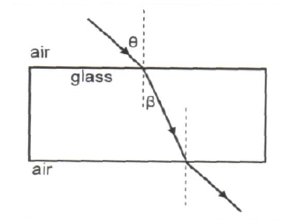
At what angle to the normal does the ray emerge from the glass block?
- β
- θ
- 90º – θ
- 90º – β (2)
1.7 When total internal reflection takes place, the size of the angle of reflection is …
- smaller than the angle of incidence.
- greater than the angle of incidence.
- the same as the angle of incidence.
- the same as the critical angle. (2)
1.8 The magnification of a convex lens is 2. The distance of the object from the optical centre is 30 mm. The image distance is …
- 15 mm.
- 28 mm.
- 30 mm.
- 60 mm. (2)
1.9 The phenomenon by which a light ray strikes a surface and goes back into the medium of incidence is known as …
- reflection.
- diffraction.
- refraction.
- dispersion. (2)
1.10 If an object is placed between the focal point and the convex lens, the image formed will be …
- enlarged, inverted and virtual.
- enlarged, upright and virtual.
- smaller, inverted and real.
- smaller, upright and real. (2)
[20]
QUESTION 2 (Start on a NEW page.)
Organic chemistry is the chemistry of organic molecules divided into homologous series which are identified by the functional groups. It is this branch of chemistry that is applied in the industry and in medicine. Pharmaceuticals used in cancer treatment and other conditions follow organic synthesis.
2.1 Explain the term homologous series. (2)
2.2 Define the term functional group. (2)
2.3 Study the organic compounds listed in the table below, labelled A‒F and answer the questions that follow.
| A | B | C |
| But-2-ene | 1-chlorobutan-2-one | Propan-1-ol |
| D | E | F |
| 3-bromo-5- methylhexene | 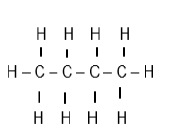 | 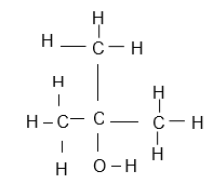 |
Write down the LETTER that represents:
2.3.1 An alkane (1)
2.3.2 A primary alcohol (1)
2.4 Write down the IUPAC name of structures:
2.4.1 E (2)
2.4.2 F (2)
2.5 Draw the structural formula of:
2.5.1 A (2)
2.5.2 C (2)
2.5.3 An isomer of E (2)
2.6 Define the term positional isomer in words. (2)
2.7 To which homologous series does compound B belong? (1)
2.8 Draw the structural formula of the functional group of compound B. (2)
[21]
QUESTION 3 (Start on a NEW page.)
Rubber is a naturally occurring compound. The diene, 2-methyl-1,3-butandiene, is one of the repeating units found in rubber.
Over 20 million families depend on rubber cultivation for their livelihood. Tens of thousands of hectares of tropical forests have been cleared to make way for rubber plantations.
Chemists have been able to combine other dienes to obtain synthetic rubbers. Some rubber products include latex products such as hand gloves, raincoats and other products used in the fight against HIV/Aids and COVID-19.
The world’s largest use of rubber is in the production of tyres, and most tyres contain both natural rubber, which withstands heat better, and one or more kinds of synthetic rubber.
3.1 Define the term polymer in words. (2)
3.2 Is but-2-ene an example of a saturated or an unsaturated hydrocarbon? Give a reason for the answer. (3)
3.3 Write down the structural formula of 2-methylbutane. (2)
3.4 With regard to the environment, name TWO disadvantages of rubber and the production of rubber. (2)
3.5 With regard to human life, name TWO benefits of rubber and the production of rubber. (4)
[13]
QUESTION 4 (Start on a NEW page.)
In the table below FIVE alcohols, represented by the letters A–E, are listed.
| A | Methanol | B | Ethanol |
| C | Propan-1-ol | D | Butan-2-nol |
| E | 2-methylpropan-2-ol |
4.1 Write down the letter that represents a secondary alcohol from the above list. (2)
4.2 Letter E represents 2-methylpropan-2-ol. Write down the following for this alcohol:
4.2.1 The structural formula (2)
4.2.2 The letter of an alcohol that represents its structural isomer (2)
4.3 Viscosity is a measure of a fluid's resistance to flow. Learners conduct an investigation to compare the viscosities of the first three alcohols (A–C) in the above table. They use the apparatus shown below.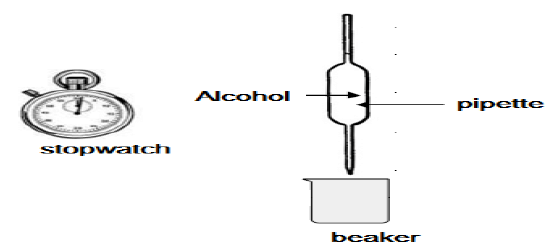
The learners use a stopwatch to measure the time it takes a FIXED VOLUME of each of the alcohols to flow from the pipette. They record this flow time, which is an indication of the viscosity of each alcohol, as given in the table below.
| ALCOHOL | FLOW TIME (s) | |
| A | Methanol | 4,0 |
| B | Ethanol | 7,9 |
| C | Propan-1-ol | 14,3 |
4.3.1 Which ONE of the alcohols A, B, or C has the highest viscosity? Use the data in the table to give a reason for the answer. (2)
4.3.2 Refer to the intermolecular forces of the three alcohols A, B and C to explain the trend in viscosities as shown in the table. (3)
4.3.3 Lubricants reduce friction. Which ONE of alcohols, A, B or C, will be the best lubricant? Explain your answer. (3)
4.3.4 Define the term vapour pressure in words. (2)
4.4 Which ONE of 2-methylpropan-2-ol and butan-2-ol has a higher viscosity? Explain the answer. (3)
[19]
QUESTION 5 (Start on a NEW page.)
In the flow diagram below X, Y and Z represent three different types of organic reactions. P represents an organic compound.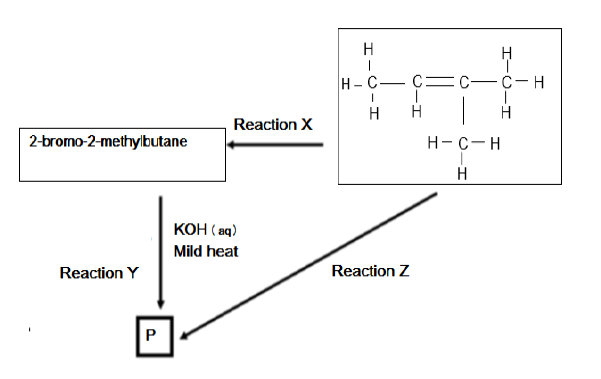
5.1 Name the type of reaction represented by reaction X. (2)
5.2 State TWO reaction conditions required for reaction X to take place. (2)
5.3 Reaction Y represents a substitution reaction. Write down the structural formula of compound P formed in the reaction. (2)
5.4 Apart from the organic reactant, write down the NAME or FORMULA of the other reactant needed in reaction Z (2)
5.5 Write down a balanced chemical reaction for the formation of compound P using structural formulae. (4)
5.6 Name the type of reaction represented by reaction Z. (2)
5.7 A car engine at a Shell Garage in Willowvale was running when a debate started between learners of a Technical Sciences class in a particular secondary school in that area. They noticed smoke coming out of the exhaust system and liquid droplets at the back of the exhaust system. One learner claimed that hexane was burning inside. They eventually reached an agreement about their observations.
5.7.1 Give the names of the products of combustion of hexane. (2)
5.7.2 Write down a balanced equation for the complete combustion of hexane. (4)
[20]
QUESTION 6 (Start on a NEW page.)
Hexanoic acid is responsible for the unique odour associated with goats. When it reacts with alcohol X, ethylhexanoate, which is used commercially as a fruit flavour is formed.
Learners set up the apparatus shown below to prepare ethylhexanoate in a laboratory.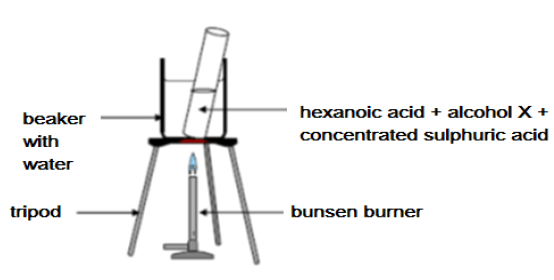
6.1 Write down the IUPAC name of alcohol X. (2)
6.2 What is the role of the sulphuric acid in the above reaction? (2)
6.3 Use structural formulae to write down a balanced equation for the preparation of ethyl hexanoate. (4)
6.4 Give ONE reason why the test tube and its contents are heated in a water bath and not directly over the flame. (2)
6.5 Write down ONE use of esters in the food manufacturing industry. (2)
[12]
QUESTION 7 (Start on a NEW page.)
Below is part of a student’s revision notes about waves. The notes contain several scientific errors.
WAVES
|
7.1 The above statements are incorrect. Identify the errors and show how each error can be corrected. (6)
7.2 The diagrams below show the refraction of light as it passes from air into different media, benzene, diamond and water.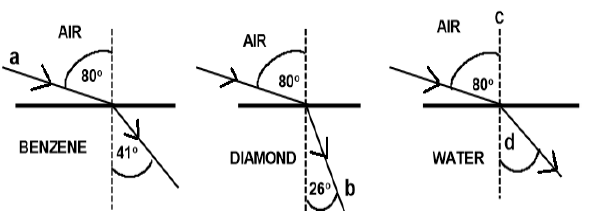
Write down the names for the following:
7.2.1 Light ray a (1)
7.2.2 Light ray b (1)
7.2.3 Dashed line c (1)
7.2.4 The angle labelled d (1)
7.3 Define the term refraction in words. (2)
[12]
QUESTION 8 (Start on a NEW page.)
The diagram below shows a ray of light travelling inside a denser medium and did not leave the denser medium to enter the less dense medium. This is a phenomenon in waves.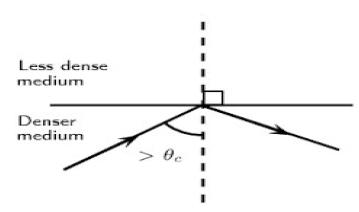
8.1 Name the phenomenon illustrated in the diagram above. (1)
8.2 Describe this phenomenon in words. (2)
8.3 What TWO conditions must be satisfied in order for the light ray to behave as it does in the diagram above? (2)
8.4 Give TWO essential applications of this phenomenon. (2)
8.5 Define the term critical angle in words. (2)
8.6 State the Law of Reflection in words. (2)
8.7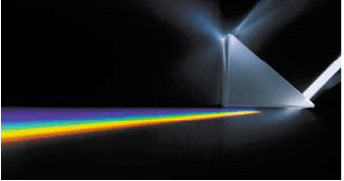
The same light ray is now passed in a similar medium as illustrated above but another phenomenon is demonstrated as is observed in this diagram.
8.7.1 Name the phenomenon demonstrated in this diagram. (1)
8.7.2 Describe this phenomenon that is demonstrated in the diagram. (2)
8.8 Sipho uses a magnifying glass with a focal length of 4 cm to view an ant. He finds that he gets a clear image of the ant when it is 2,5 cm away from the lens. The ant is 1 cm in size.
8.8.1 What type of lens does a magnifying glass represents? (1)
8.8.2 Define focal length. (2)
8.8.3 Give THREE properties of the image formed if ray diagrams were used. (3)
[20]
QUESTION 9 (Start on a NEW page.)
Albert Einstein, Geiger and Gamma suggest that light can act as a particle or as a wave. The diagram below represents radiations of the electromagnetic spectrum.
| Infrared | Radio waves | X-ray | Microwave | Gamma rays | Ultraviolet rays | Visible light |
9.1 Arrange the spectrum in order of increasing frequency. (2)
9.2 In which radiation will a photon have the highest energy? (1)
9.3 Explain the answer to QUESTION 9.2. (2)
9.4 Define the term electromagnetic waves in words. (2)
9.5 Calculate the energy of a photon of an electromagnetic radiation with a wavelength of 540 nm. (5)
9.6 How will the energy of a photon with a wavelength of 520 nm compare with that calculated in QUESTION 9.5? (Write only GREATER THAN, SMALLER THAN or EQUAL TO.) (1)
[13]
TOTAL: 150
DATA TABLES FOR TECHNICAL SCIENCE GRADE 12
PAPER 2
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,01 x 105 Pa |
Standard temperature | Tθ | 273 K |
Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT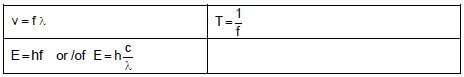
TABLE 3: ELECTROCHEMISTRY
Eθcell = Eθcathode - Eθanode |
TABLE 3: THE PERIODIC TABLE OF ELEMENTS
Technical Sciences Paper 1 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
- Write your FULL NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of EIGHT questions. Answer ALL the questions in the ANSWER BOOK.
- Start each question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 A bag is placed on a frictionless floor in a bus travelling due west at a constant velocity. When the bus stops suddenly, the bag will ...
- not move.
- move due east.
- move due west.
- move due south. (2)
1.2 Which ONE of the following is always in the same direction as the acceleration of a body?
- Velocity
- Work done
- Momentum
- Rate of change of momentum (2)
1.3 A block of mass m pulled over a frictionless surface with a force of F has an acceleration of a m.s-2.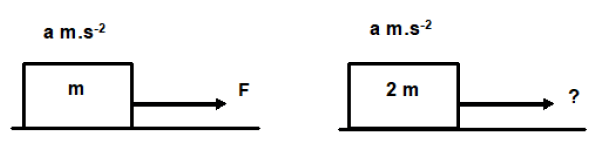
What would be the magnitude of the force required to accelerate a block of mass 2m at a m.s-2?
- ½F
- F
- 2F
- 3F (2)
1.4 Two blocks, P and Q, initially at rest, have a spring compressed between them. The spring is released and the blocks move away from each other. IGNORE FRICTION.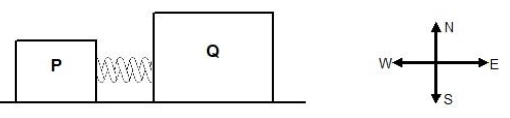
The magnitude of the momentum of block P after the spring is released is x kg.m.s-1.
Which ONE of the following CORRECTLY gives the total momentum of the system, before the spring is released and the momentum of block Q after the spring is released?
Total momentum of the system before the spring is released | Momentum of block Q after the spring is released | |
A | 0 | x due east |
B | x due west | 0 |
C | x due east | x due west |
D | 0 | x due west |
(2)
1.5 The work done to move an object over a distance by a force F which is applied 60° to the horizontal is W.
The work done to move the object through the same distance by the force F acting horizontally to the surface is …
- ½W.
- W.
- 2W.
- 3W. (2)
1.6 Consider the following statements regarding fluid pressure:
- Fluid pressure is directly proportional to the depth
- Fluid pressure is independent of the size or shape of the container
- Fluid pressure is inversely proportional to the density of the fluid
Which ONE of the following is CORRECT regarding fluid pressure?
- (i) and (ii)
- (ii) and (iii)
- (i) and (iii)
- (i), (ii) and (iii) (2)
1.7 A force, which changes the shape and size of a body, is … force.
- normal
- frictional
- restoring
- deforming (2)
1.8 The maximum force that can be applied on a body so that the body regains its original form completely on removal of the force is …
- strain.
- stress.
- tension.
- elastic limit. (2)
1.9 The equivalent unit of Farad (F) is …
- C∙V.
- C∙V-1.
- C∙V-2.
- C∙V2. (2)
1.10 In the circuit given below the resistance of Y is twice that of X.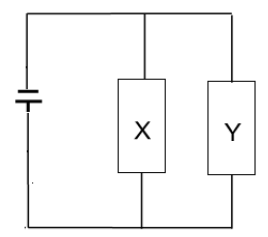
If the current in X is 1 A, then the current in Y will be …
- ½ A.
- 1 A.
- 2 A.
- 4 A. (2)
[20]
QUESTION 2 (Start on a new page.)
A block is pulled over a rough surface XY by means of a force F. The block moves at a constant velocity of 5 m.s-1 along part XY.
When the block moves past Y the force F is removed.
Part YZ is frictionless.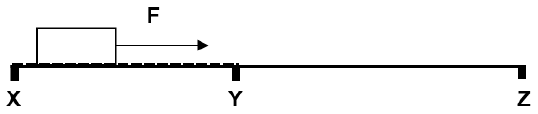
2.1 Draw a free body diagram of all the forces acting on the block when the block is on part YZ. (2)
2.2 State Newton’s first law of motion in words. (2)
2.3 Describe the motion of the block on part YZ. (2)
[6]
QUESTION 3 (Start on a new page.)
Two blocks of masses 30 kg and 20 kg connected by a light inextensible string are pulled along a rough surface by force F, applied at an angle 30° to the horizontal.
The tension T on the string is 50 N and coefficient of kinetic friction is 0,2 for both blocks.
Assume that the system is isolated.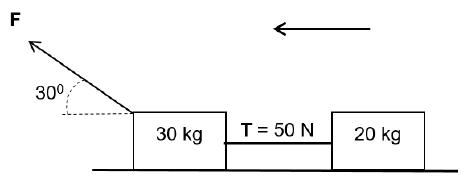
3.1 State Newton’s second law of motion in words. (2)
3.2 Define the term isolated system. (2)
3.3 Draw the free body diagram of all the forces acting on the 30 kg block. (5)
3.4 Calculate the:
3.4.1 Acceleration of the blocks. (4)
3.4.2 Magnitude of force F that is applied on the 30 kg block. (5)
3.5 Force F is now applied downward on the 30 kg block at an angle 30° to the horizontal as shown in the diagram.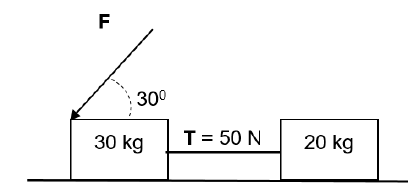
How would the following quantities change?
(State INCREASES, DECREASES or REMAINS THE SAME.)
3.5.1 Acceleration of the system (1)
3.5.2 Tension T on the string (1)
3.6 Explain the answer to QUESTION 3.5.1. (2)
[22]
QUESTION 4 (Start on a new page.)
A 5 kg block moving due east with a velocity of 4 m∙s-1 collides with a stationary 3 kg block.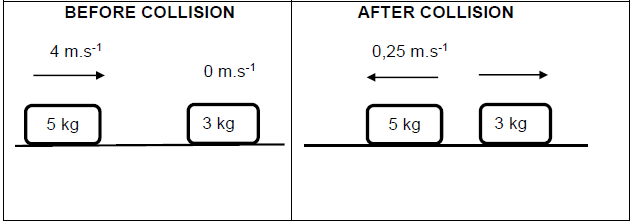
Immediately after the collision, the 5 kg block moves due west with a velocity of 0,25 m.s-1 and the 3 kg block moves with a constant velocity due east as shown in the diagram below.
The collision is inelastic.
4.1 Define the term momentum. (2)
4.2 Which quantity (MOMENTUM or KINETIC ENERGY) is conserved during this collision? (1)
4.3 Calculate the velocity of the 3 kg block after collision. (4)
4.4 Name and state the law used in the calculation in QUESTION 4.3 above. (3)
[10]
QUESTION 5 (Start on a new page.)
A car with a mass of 1 000 kg travelling due east, collides head-on with a truck with a mass of 5 000 kg moving at 20 m·s-1 due west. The force exerted by the truck on the car during the collision is 100 000 N and the collision lasts for 0,4 s.
After the collision, the car and the truck move together. Ignore the effects of friction.
5.1 What is the magnitude and direction of the force exerted by the car on the truck during the collision? (2)
5.2 Name and state the law used to answer QUESTION 5.1 above. (3)
5.3 Define the term impulse. (2)
5.4 Calculate the velocity of the car after the collision. (4)
[11]
QUESTION 6 (Start on a new page.)
6.1 A crate of bricks with a mass of 50 kg is lifted up by a motor which is placed at the top of a building as shown in the diagram. The force exerted by the motor is F and the block moves upward with a constant velocity of 5 m.s-1.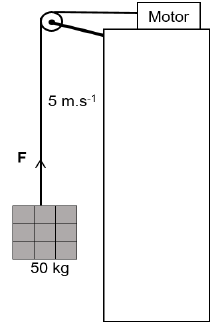
6.1.1 Define the term work. (2)
6.1.2 Name all the forces acting on the crate of bricks. (2)
6.1.3 What is the net force acting on the crate of bricks? Give a reason for your answer. (2)
6.1.4 Calculate the power generated by the motor. (4)
6.2 A brick with a mass of 0,5 kg is thrown downwards with a velocity of 10 m.s-1 from height h metres above the ground. When the brick is ⅔h metres above the ground, its velocity is 20,34 m.s-1. (IGNORE AIR FRICTION.)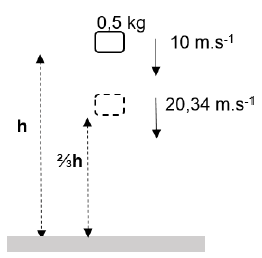
6.2.1 State the law of conservation of mechanical energy. (2)
6.2.2 Calculate the mechanical energy of the brick. (6)
6.2.3 Calculate the velocity of the brick just before it touches the ground. (3)
6.3 An object of mass 20 kg is pulled along a straight horizontal road to the right without being lifted. The force diagram below shows all the forces acting on the object.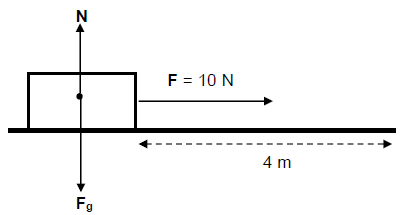
The applied force F is 10 N and the object is displaced through a distance of 4 m.
6.3.1 What is the value of the work done by the normal force (N)? Explain. (3)
6.3.2 Calculate the work done by force F. (3)
[27]
QUESTION 7 (Start on a new page.)
7.1 What is a perfectly elastic body? (2)
7.2 Define the term stress. (2)
7.3 A stress vs strain graph for steel and tungsten is given below.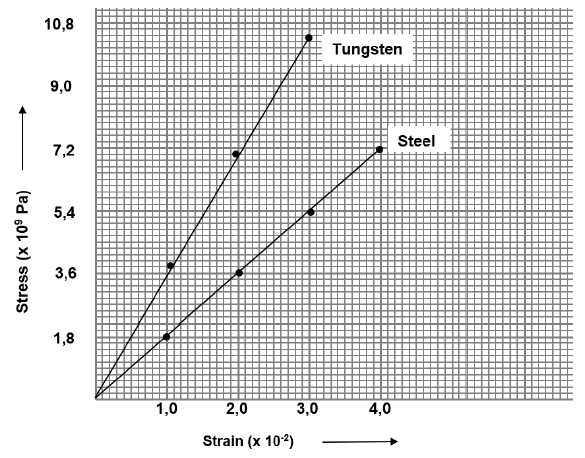
Answer the following questions using the above graph.
7.3.1 Write down the relationship between stress and strain. (2)
7.3.2 Write down the name of the law that states the above relationship between stress and strain. (1)
7.3.3 Which ONE of the materials has the highest modulus of elasticity? Explain. (2)
7.3.4 Calculate the modulus of elasticity of steel. (3)
7.4 The viscosity of three different substances at 25 °C are given in the table below.
Substance | Viscosity (Pa.s) |
Water | 8,94 x 10-4 |
Blood | 3,5 x 10-3 |
Motor oil SAE 10 | 6,5 x 10-2 |
7.4.1 Define viscosity. (2)
7.4.2 Which ONE of the above substances flows the fastest? Explain your answer. (2)
7.4.3 How does the increase in temperature affect the viscosity? (2)
7.5 A simple diagram of hydraulic brakes is given below. The area of piston A is 284 × 10-6 m2 and piston B is 507 × 10-6 m2. The force applied on the brake pedal is 1 765 N.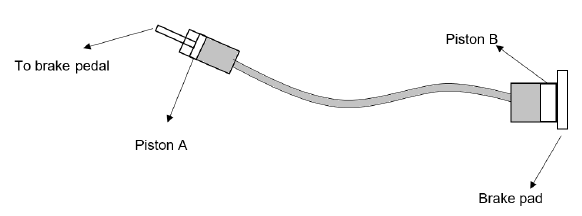
7.5.1 State Pascal’s law in words. (2)
7.5.2 Calculate the force exerted by piston B on the brake pad. (4)
7.5.3 What would happen to the pressure on the brake pad if the area of piston B is increased? (1)
Write down only INCREASES, DECREASES or REMAINS THE SAME
7.5.4 Write down TWO uses of hydraulic systems other than hydraulic brakes. (2)
[27]
QUESTION 8 (Start on a new page.)
8.1 Semiconductor Ge is doped with the element phosphorus (P).
8.1.1 What is doping? (2)
8.1.2 What is the number of valence electrons in phosphorus? (1)
8.1.3 What type (n-type or p-type) of semiconductor is produced in the above process? (2)
8.1.4 What is the difference between n-type and p-type material? (2)
8.2 Each plate of a parallel plate capacitor has an area of 10 cm2. The plates are separated by a distance of 1 cm, with air occupying the space between the plates. The capacitor is connected to a 12 V DC supply.
8.2.1 Calculate the magnitude of charge on each plate. (6)
8.2.2 What is the net charge on the capacitor? (1)
8.2.3 Name TWO factors that can affect the capacitance of a capacitor. (2)
8.3 An electric kettle with resistance 26 Ω and a microwave oven with resistance 52 Ω are connected in parallel and the combination is connected across a source of voltage 230 V as shown in the diagram.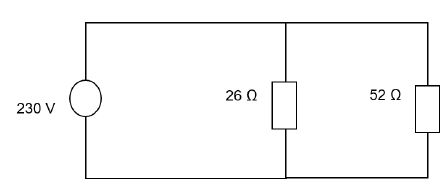
8.3.1 Define power. (2)
8.3.2 Calculate the total resistance of the circuit. (3)
8.3.3 Calculate the power developed across the electric kettle. (3)
8.3.4 How much heat is produced in the electric kettle in 2 minutes? (3)
[27]
TOTAL: 150
DATA FOR TECHNICAL SCIENCES GRADE 12
PAPER 1
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Acceleration due to gravity | g | 9,8 m·s-2 |
| Coulomb's constant | k | 9,0 x 109 N·m2·C-2 |
| Permittivity of free space | ε0 | 8,85 x 10-12 F.m-1 |
TABLE 2: FORMULAE
FORCE
WORK, ENERGY AND POWER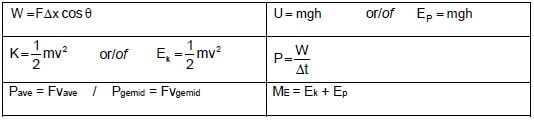
ELASTICITY, VISCOSITY AND HYDRAULICS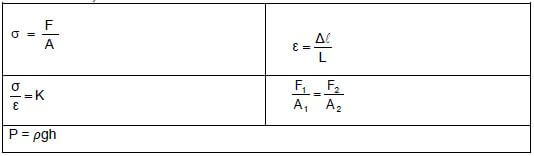
ELECTROSTATICS
CURRENT ELECTRICITY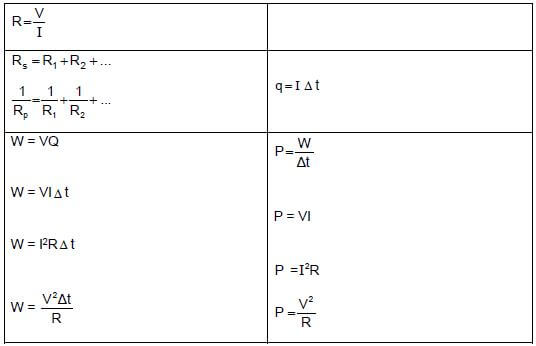
ELECTROMAGNETISM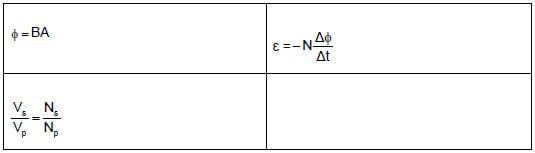
Physical Science Paper 2 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
MEMORANDUM
QUESTION 1
1.1 D (2)
1.2 C (2)
1.3 A (2)
1.4 A (2)
1.5 C (2)
1.6 B (2)
1.7 B (2)
1.8 D (2)
1.9 D (2)
1.10 B (2) [20]
QUESTION 2
2.1
- A series of organic compounds that can be described by the same general formula
OR
A series of organic compounds in which members differ by the number of – CH2 units (2)
2.2 A compound that contains carbon and hydrogen (atoms) only (2)
2.3.1 CnH2n+2 (1)
2.3.2 4-ethyl -2,2-dimethyl hexane
Marking criteria
- Hexane
- Methyl/Metiel and
- whole name correct 3/3
Deduct 1 mark for any error, hyphens omitted, incorrect sequence etc (3)
2.4.1 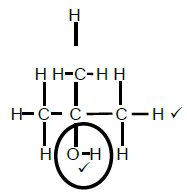 (2)
(2)
Marking criteria
- Hexane
- Methyl and ethyl
- whole name correct 3/3
Deduct 1 mark for any error, hyphens omitted, incorrect sequence etc
2.4.2 Butan-2-one OR 2-butanone (2)
Marking criteria
- Functional group and correct position g pent-2-one (1/2)
- Whole name correct (2/2)
2.4.3
- The functional group can only be in position 1
OR
Side chain / branch / methyl group can only be in position 2 (1)
2.5.1 Polymerisation (1)
2.5.2 Contains single bonds only (1 or 0) (1)
2.5.3 Use in plastics (toy cars) (1)
[16]
QUESTION 3
3.1 The temperature at which the vapour pressure of a liquid equals the atmospheric/external pressure. (2)
3.2 To ensure a fair test /To ensure there is one independent variable (1)
3.3
- Hexane has London forces (only)
Pentanal has dipole-dipole forces (and London forces) - Dipole-dipole forces are stronger ( than the london forces in hexane)
OR London forces are weaker (than the Dipole-dipole forces in pentanal) - More energy is required to overcome the intermolecular /Dipole-dipole forces in Pentanal (4)
3.4 Higher than (1)
3.5
- Chain isomer of pentan-2-ol (2-methylbutan-2-ol) has a shorter chain length/ smaller surface area than pentan-2-ol
- London forces in the isomer of pentan-2-ol (2-methylbutan-2-ol) will be weaker (than that of pentan-2-ol).
OR - Pentan-2-ol has a larger chain length/ surface area than its chain isomer
- London forces in butan-2-ol is stronger (than that of the isomer of pentan-2-ol (2-methylbutan-2-ol) (3)
3.6 2 C6H14 + 19 O2 → 12 CO2 + 14 H2O
Marking criteria
- Reactants
- Products
- Balancing (3)
[14]
QUESTION 4
4.1.1 Substitution /Hydrolysis (1)
4.1.2 Butan-2-ol OR/OF 2-butanol (2)
4.2 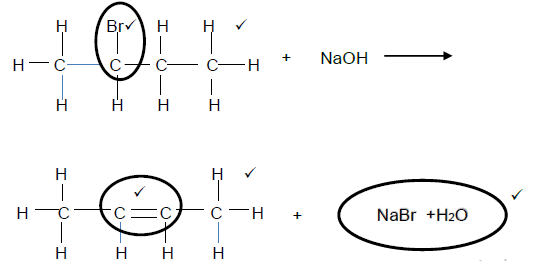 (6)
(6)
Balancing
Marking criteria
For organic reagents
- Whole structure correct(2/2)
- Functional group correct (1/2)
4.3
4.3.1 Hydrogenation (1)
4.3.2 Platinum/Palladium/Nickel (1)
4.3.3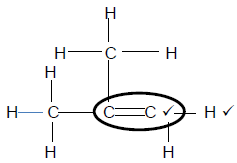
Marking criteria
For organic reagents
- Whole structure correct (2/2)
- Functional group correct (1/2) (2)
4.4
4.4.1 Esterification/Condensation (1)
4.4.2 Heat/Add a catalyst/H2SO4 (1)
4.4.3 Water/H2O (1)
4.4.4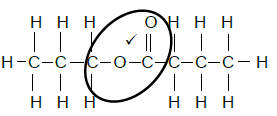
Marking criteria
- Whole structure correct (2/2)
- Functional group correct (1/2)
- Name correct (2/2)
Propyl butanoate (4)
[20]
QUESTION 5
5.1 The change in concentration of reactants or products per unit time.(2)
5.2 Carbon dioxide/CO2 (1)
5.3 Stopwatch (1)
5.4
- Rate = -Δ m/Δt
Rate= –(200 -184,80) / (5 – 0)
Rate = 3,04 (g∙min-1)
Accept:
Rate = (184,80 –200) / (5 – 0) Rate = (200 –184,80) /(5 – 0)
= - 3,04 (g∙min-1) Rate = 3,04 (g∙min-1) (3)
5.5
- mCO2 produced = 200- 184,80
= 15.2 g
nCO2 = m/M
= 15,2 /44
= 0,35 mol
nCaCO3 reacting = 0.35 mol
= 0.35 x 100
= 35 g
Marking guidelines
- Mass of CO2
- Formula n = m/M
- Substitution of 44 into n = m/M
- Use of ratio CaCO3: CO2 (1:1)
- Substitution of 100 into n = m/M
- Final answer
(Ratio) mCaCO3 reacting= n.M (6)
5.6.1 Temperature (1)
5.6.2 B (1)
5.6.3
- Higher temperature increases average kinetic energy of the particles increases.
- More particles have sufficient kinetic energy (to collide effectively) / More particles have Ek greater or equal to Ea
- More effective collisions per unit time (3)
5.7.1
- What effect will the concentration (of a substance) have on the rate of reaction?
OR - What is the relationship between reaction rate and concentration?
OR - How does concentration affect reaction rate?
Marking criteria
For organic reagents
- Independent variable and dependent variable correct
- In the form of a question (2)
5.7.2 LOWER THAN (1)
5.7.3 EQUAL TO(1)
5.8 The same amount of CaCO3 (the limiting reagent) is used in both experiments (2)
[24]
QUESTION 6
6.1 Reaction in which products can be converted back to reactants (2)
6.2 FORWARD REACTION(1)
6.3 No
- The rate of forward reaction is equal to the rate of reverse reaction /Reaction reached equilibrium (3)
6.4.1 Increases (1)
6.4.2 Remains the same (1)
6.4.3 Increases (1)
6.5
- The amount of HI remains constant.
- The volume decreases
- The concentration increases according to c = n/V (2)
6.6.1 High yield Kc is large OR Kc >1 (2)
6.6.2 EXOTHERMIC
- The value Kc decreases with an increase in temperature.
- As temperature increases, the [prdoducts] decreases
- Reverse reaction is favoured by an increase in temperature
OR - The value Kc increases with a decrease in temperature.
- As temperature decreases the [products] increases
- Forward reaction is favoured by a decrease in temperature (4)
6.7.1
- Kc = [HI]2/[H2].[I2]
50,3 = [HI]2/(0.46)(0,39)
[HI] = 3 mol.dm-3 (4)
6.7.2 POSITIVE MARKING FROM 6.7.1
OPTION 1 : CALCULATIONS USING NUMBER OF MOLES
Marking Criteria for Mole Option
- Multiplication/ van c equilibrium by/ met 0,5 dm3 for/ vir I2 ,H2 and HI
- Calculations of moles of HI reacting
- Using mole ratio 2 n(HI) = n(H2) = n (I2) reacting
- Calculation of (H2) initial and n (I2) initial (Δn + nequilibrium)
- Multiplication / van n (I2) by 2 to find n (HI) theoretical
- Substitution into Yield = nproduced x 100
- Final answer (RANGE : 78,95% – 79,37%)
CALCULATION USING MOLES
H2 | I2 | 2HI | |
ni | 0,98 | 0,945 | 0 |
Δn | 0,75 | 0,75 Ratio | 1,5 |
nequilibrium | 0,23 | 0,195 | 1,5 (x 0,5 dm3) |
cequilibrium | 0.46 | 0,39 | 3 |
I2 is the limiting reagent
n (HI) theoretical = 2 (n I2 initial) = 2 x (0,945) = 1,89 mol
% Yield = nproduced X 100 = 1,5/1,89 x 100 = 79,37 %
H2 used as limiting reagent Max 3/7
OPTION 2: CALCULATION USING CONCENTRATIONS
Marking Criteria for concentration Option
- Calculations of cHI reacting
- Using mole ratio 2 c(HI) = c(H2) = c(N2) reacting
- Calculation of cH2 initial and cI2 initial (Δc + cequilibrium )
- Multiplication of cI2 by 2 to find vind nHI theoretical
- Substitution into Yield = n
HIproduced x 100 - Final answer
(RANGE: 78,95% -79,37%)
H2 | I2 | 2HI | |
ci | 1,96 | 1,89 | 0 |
Δc | 1,5 | 1,5 (Ratio) | 3 (cHI equil) |
cequilibrium | 0.46 | 0,39 | 3 |
I2 is the limiting reagent
C (HI) theoretical = 2 x 1,89 = 3,78 mol.dm-3
% Yield = cproduced x 100 =3/3,78 x 100 =79,37% (7)
H2 used as limiting reagent Max 3/7
[28]
QUESTION 7
7.1.1 An acid is a substance that donates protons /H+ions (2)
7.1.2 H2PO4 (1)
7.1.3
- Reaction I : Reverse reaction it accepts a proton (H+) / acts as a base
- Reaction II: Foward reaction donates a proton (H+)/ act as an acid. (2)
7.1.4 HPO42
- The conjugate base of a weak acid
- lower Ka value is the stronger base (3)
7.2.1 Reaction of a salt with water (2)
7.2.2 C2HO4 (2)
7.2.3 (Excess) OH- ions/hydroxide ions are produced (2)
7.3.1 A strong base undergoes complete ionisation /disociation (2)
7.3.2 | OPTION 1 / OPSIE 1 | OPTION 2 / OPSIE 2 | |
Kw = [H3O+][OH-] | pOH = -log[OH-] | ||
1x10-14 = [H3O+](0,5) | pOH = - log (0,5) | ||
[H3O+] = 2x10-14 mol∙dm-3 | pOH = 0,30 | ||
pH = -log[H3O+] | pH + pOH = 14 | ||
pH = - log(2x10-14) | pH + 0,30 = 14 | ||
pH = 13,70 | pH = 13,70 | (5) |
7.3.3 OPTION 1
- caVa = na
cbVb nb
ca(25) = 1
(0,5)(24) 2
ca = 0,24 mol∙dm-3
c = m
MV
0,24 = 7,56
(90+18x)(0,25)
x = 2
OPTION 2 :
Marking guideline
- Substitution of 0,5 and 24/100 in n = cV
- Use of mole ratio in Acid: Base 1:2
- Calculating number of moles of acid in original solution
- Use of 90 in m = nM
- Calculation of mass of water of crystallization in original solution
- Calculating ration of nWater/nAcid
- Final answer
n NaOH reacting = cV= 0.5 x 24/1000 = 0.012 mol
noxalic acid reacting = ½ x 0.012 mol = 0,006 mol
noxalic acid in original solution = 250/25 x 0,006
= 0,06 mol
moxalic acid in original solution = nM = 0,06 x 90
= 5,4 g
m H2O of crystallisation in original solution = 7,56 - 5,4
= 2.16 g
n H2O crystalllisation = 2,16 /18 = 0,12 mol
x = 0,12/0,06 = 2 (7)
[28]
TOTAL 150
Physical Science Paper 1 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
MEMORANDUM
QUESTION 1:
MULTIPLE-CHOICE QUESTIONS
1.1 C (2)
1.2 C (2)
1.3 A (2)
1.4 D (2)
1.5 A (2)
1.6 B (2)
1.7 D (2)
1.8 B (2)
1.9 D (2)
1.10 C (2) [20]
QUESTION 2
2.1 When a resultant/net force acts on an object, it accelerates in the direction of the force. The acceleration is directly proportional to the force and inversely proportional to the mass of the object. (2)
2.2
| OPTION 1 | OPTION 2 |
 | |
(4)
- Mark awarded for arrow and label.
- Do not penalise for length of arrows since drawing is not drawn to scale.
- Any other additional force(s)
- If force(s) do not make contact with body.
2.3
OPTION 1 (To the right is positive) | OPTION 2 (To the right is negative) |
Fnet = ma T – Wsin θ – f = ma | Fnet = ma Wsin θ + f – T = ma |
(4)
2.4
2.4.1The kinetic friction force depends on the angle of the inclined to the horizontal and nature of the surface. (2)
2.4.2
Positive marking from Question 2.3 | Marking criteria |
For 15 kg block For 22 kg block ∴ a = 2,278 m.s-2 ? | Fnet = ma Answer? |
(6) [18]
QUESTION 3
3.1
UPWARD POSITIVE | UPWARD NEGATIVE |
vf = vi + a∆t ? | vf = vi + a∆t ? |
3.2
OPTION 1 | |
UPWARD POSITIVE | UPWARD NEGATIVE |
∆y = vi ∆t + 1 a∆t2 ? | ∆y = vi∆t+ 1 a∆t2 ? |
OPTION 2 | |
Positive marking from Question 3.1 | |
UPWARD POSITIVE | UPWARD NEGATIVE |
vf2= vi2 + 2a∆y ? | vf2 = vi2 + 2a∆? ? |
OPTION 3 | |
Positive marking from Question 3.1 | |
UPWARD POSITIVE | UPWARD NEGATIVE |
∆y = vf + vi Δt | ∆y = vf + vi Δt |
(4)
3.3 | OPTION 1 | ||
UPWARD POSITIVE | UPWARD NEGATIVE | ||
vf2= vi2 + 2a∆y | vf2= vi2 + 2a∆y | ||
OPTION 2 | (3) | ||
UPWARD POSITIVE | UPWARD NEGATIVE | ||
∆y = vi∆t+ 1 a∆t2 | ∆y = vi∆t+ 1 a∆t2 | ||
3.4 | Positive marking from Question 3.2 | (4) | |
UPWARD POSITIVE | UPWARD NEGATIVE | ||
Fnet.∆t = m(vf - vi) | Fnet. ∆t = m(vf - vi) | ||
3.5 | OPTION 1 | |||
Positive marking from Question 3.1 and 3.3 | ||||
UPWARD POSITIVE | UPWARD NEGATIVE | |||
vf2= vi2 + 2a∆y | vf2= vi2 + 2a∆y | |||
OPTION 2 | (5) | |||
Wnet = ∆Ek | ||||
[19] | ||||
[19]
QUESTION 4
4.1 In an isolated system total linear momentum is conserved. (2)
4.2
4.2.1
- MEi = MEf
mgh1 + ½ mvi2 = mgh2 + ½ mvf2
0 + ½ x 2,005 x vi2 = 2,005 x 9,8 x 0,06 + 0
vi = 1,08 m.s-1
∑pi = ∑pf Any one
mblockviblock + mbulletvibullet = (mblock + mbullet) vf
0 + 0,005 x vibullet = 2,005 x 1,08
vbullet = 433,08 m.s-1 right (5)
4.2.2
- Positive marking from Question 4.2.1
∑pi = ∑pf
(mgun + mbullet)vi = mgunvfgun + mbulletvfbullet = Any one
0 = 5 x vf(gun)+ 0,005 x 433,08
vf(gun= - 0,43 m.s-1
vf(gun = 0,43 m.s-1 left (3)
[10]
QUESTION 5
5.1 In an isolated system the total mechanical energy is conserved. (2)
5.2
- MEi = MEf
(Ep + Ek)A = (Ep + Ek)B
mgh1 + Ek(A) = mgh2 + Ek(B)
55 x 9,8 x 15 + 0 = 0 + Ek(B) Any one
Ek(B) = 8085 J (3)
5.3
- The net work done on an object is equal to the object’s change in kinetic energy.
OR - The work done by a net force is equal to the object’s change in kinetic energy. (2)
5.4
5.4.1 Positive marking from Question 5.2
- OPTION 1
Wner = ∆Ek
Wf + WFg// = ∆Ek
f x ∆x cosθ + mgsin θ ∆x cos θ = ∆Ek Any one
15 x 10 cos180o + 55 x 9,8 sin 15o x 10 cos 180o = 0 – Eki
Eki = 1545,035 J - OPTION 2
Wnc = ∆Ek + ∆Ep
Wf = ∆Ek + ∆Ep
f x ∆x cosθ = ∆Ep + ∆Ek Any one
15 x 10 cos180o = [55 x 9,8 x (10 sin15o)] - 0 + 0 - Eki
-150 = 1395,035 - Eki
Eki = 1545,035 J - OPTION 3
Wner = ∆Ek
Wf + WFg = ∆Ek Any one
f x ∆x cosθ + mg ∆xcos θ = ∆Ek
15 x 10 cos180o + 55 x 9,8 x 10 cos (90 + 15) = 0 – Eki
Eki = 1545,035 J (4)
5.4.2
OPTION 1/OPSIE 1 | OPTION 2/OPSIE 2 |
Wnet = ∆Ek | Wnc = ∆Ek + ∆Ep |
(5) [16]
QUESTION 6
6.1 Doppler effect (1)
6.2 AWAY. The observed frequency is less than the source frequency. (2)
6.3 As the listener’s velocity increases, the observed frequency decreases. (2)
6.4 The intercept on the vertical axis represents the frequency of the source, fs. (2)
6.5
OPTION 1 | OPTION 2 |
Gradient = 492 - 470 ? | fL = v ±vL fs ? |
OPTION 3 |
fL = v ±vL fs ? |
(5)
6.6
OPTION 1 | OPTION 2 | OPTION 3 |
v = fλ | v = fλ | v = fλ |
(3) [15]
QUESTION 7
7.1
- n = Q
qe
n = 2 x10-6
1,6 x10-19
n = 1,25 x 1013 (3)
7.2
 (3)
(3)
Criteria for marking
- Correct shape
- Direction of electric field
- Lines not crossing each other
7.3
7.3.1
- vf = vi + a∆t
6,25 x 103 = 0 + 2 x 10-3 a Any one
a = 3,125 x 106 m.s-2
Fnet = ma
Fnet = 5 x 10-6 x 3,125 x 106
Fnet = 15,625 N
Enet = Fnet
q
Enet = 15,625
2 x10-6
Enet = 7,81 x 106 N.C-1 (6)
7.3.2 | Positive marking from Question 7.3.1 | ||
OPTION 1 | OPTION 2 | ||
E = kQ ? | vf 2 = vi2 + 2a Δx ? | ||
Using equations of motion | |||
OPTION 3/OPSIE 3 | OPTION/OPSIE 4 | ||
Δx = ½(vf + vi) Δt ? | Δx = vi Δt + ½ a Δt² ? | ||
7.3.3 | Positive marking from Question 7.3.1 and 7.3.2 |
(4) | |
OPTION 1 | OPTION 2 | ||
FE = kQ1Q2 ? | FE = kQ1Q2 ? 15,625 ?= (9 x 109)(Q1)(2 x 10-6) | ||
[19] | |||
QUESTION 8
8.1 Resistance (1)
8.2
8.2.1
- Gradient = 4,2 - 0,2
24 - 0
= 1
6
= 1
emf
1 = 1
emf 6
8.2.2
Positive marking from Question 8.2.1 | |
OPTION 1 | OPTION 2 |
Intercept on vertical axis = r | ? = I (R + r ) ? |
8.3
8.3.1
OPTION 1 | OPTION 2 |
P = VI ? | P = V 2 |
8.3.2
Positive marking from Question 8.2.1en 8.2.2 | |
OPTION 1 | OPTION 2 |
P = V2 | P = V2 Rtoy car = 0,75 Ω |
(6)
8.4
- No, the external resistance will increase. Current will decrease.
- P = I2R, P ? I2 since resistance remain constant, power will decrease. (3)
[20]
QUESTION 9
9.1 (Electric) motor (1)
9.2 Electrical energy to mechanical energy (2)
9.3 CLOCKWISE (1)
9.4
- Replace the split ring with slip rings.
- Replace the cell with a resistor / ammeter / voltmeter. (2)
9.5
9.5.1
- Paverage = V 2rms
R
1500= 2302
R
R = 35,27 Ω (3)
9.5.2 | OPTION 1 | OPTION 2 | |
R = Vrms 35,27 = 2302 ? | Paverage = VrmsIrms | (4) |
[13]
TOTAL: 150
Physical Science Paper 2 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of SEVEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 Which ONE of the following factors will increase the rate of a chemical reaction by offering an alternative path of lower activation energy?
- Pressure
- Temperature
- Surface area
- Positive catalyst (2)
1.2 Structural isomers always have the same …
- carbon chain.
- functional group.
- molecular formula.
- physical properties. (2)
1.3 Which ONE of the following has the HIGHEST vapour pressure?
- Pentane
- Hexane
- Heptane
- Octane (2)
1.4 Which ONE of the following is the structural formula for the functional group of ethanoic acid?
(2)
1.5 Consider the chemical reaction given below:
X + concentrated H2SO4 → CH3CHCHCH3 + H2O
Which ONE of the following is CORRECT about reactant X?
Reactant X is a …
- tertiary alcohol.
- primary alcohol.
- secondary alcohol.
- primary haloalkane. (2)
1.6 The potential energy diagram shown below is for the hypothetical reversible reaction shown below.
X2(g) ⇌ Y2(g)
The value of ΔH (in kJ/mol) for the catalysed forward reaction is equal to …
- 23
- -23
- 58
- -58 (2)
1.7 The reaction represented by the balanced equation below reaches equilibrium in a closed container.
C(s) + CO2(g) ⇌ 2CO(g)
More C(s) and CO2(g) are added to the container at constant temperature.
How will the number of moles of CO(g) and the value of Kc be affected at equilibrium?
NUMBER OF MOLES OF CO | Kc | |
A | Increases | Increases |
B | Increases | Remains constant |
C | Remains the same | Remains the same |
D | Remains the same | Increases |
(2)
1.8 The relationship between [H3O+] and [OH-] in an aqueous solution at constant temperature is best represented by …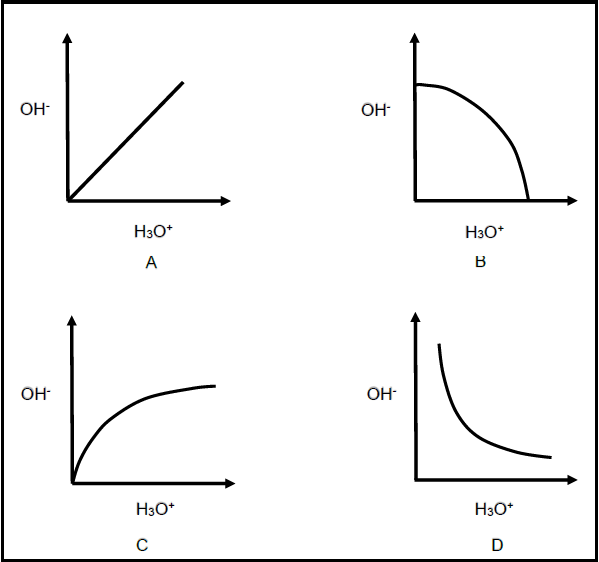
(2)
1.9 Gaseous chlorine (Cℓ2), used to disinfect water in public swimming pools reacts with water according to the following balanced equation.
Cℓ2 + H2O → HOCℓ + HCℓ
The addition of chlorine changes the pH of water in swimming pools.
Which ONE of the following substances must be added to public swimming pools periodically to increase the pH?
- KCℓ
- NH4Cℓ
- H2SO4
- Na2CO3 (2)
1.10 Consider the reaction in which magnesium powder reacts with EXCESS 50 cm3 of a 0,1 mol∙dm-3 of sulphuric acid solution.
Mg(s) + H2SO4(aq) → MgCl2 (aq) + H2(g) ΔH < 0
Which ONE of the following changes will increase the rate of production of hydrogen gas?
- Increase in pressure.
- Heating the reaction mixture.
- Using 100 cm3 of the same acid solution.
- Adding water to the reaction mixture. (2)
[20]
QUESTION 2 (Start on a new page.)
Consider compound A which is a member of homologous series of saturated hydrocarbons.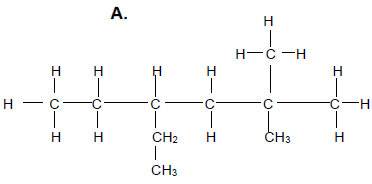
2.1 Define the term homologous series. (2)
2.2 Give a reason why compound A is classified as a hydrocarbon. (2)
2.3 For compound A write down the:
2.3.1 General formula of the homologous series to which it belongs (1)
2.3.2 IUPAC name (3)
2.4 Consider compounds P and Q shown below:
P: 2-methyl-2-propanol Q: 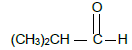
Write down the:
2.4.1 Structural formula of compound P (2)
2.4.2 IUPAC name of a FUNCTIONAL isomer of compound Q (2)
2.4.3 Give a reason why compound Q CANNOT have a POSITIONAL isomer. (1)
2.5 Polyethene is produced when many ethene monomer units join together to form a polymer according to the equation:![]()
2.5.1 Write down the type of reaction described by the underlined phrase. (1)
2.5.2 Give a reason why polyethene is regarded as saturated. (1)
2.5.3 Name ONE use of polyethene. (1)
[16]
QUESTION 3 (Start on a new page.)
The boiling points shown in the table were obtained during an investigation into the boiling points of compounds A, B and C. The compounds have a comparable molecular mass.
| Compound | Boiling point (°C) | |
| A | Hexane | 68 |
| B | Pentanal | 103 |
| C | Pentan-2-ol | 119 |
3.1 Define the term boiling point. (2)
3.2 Give a reason why the compounds used in the investigation must have a comparable molecular mass. (1)
3.3 Explain the difference in boiling points of compound A and B by referring to the TYPE and STRENGTH of intermolecular forces and energy involved. (4)
3.4 How will the vapour pressure of a CHAIN isomer of compound C compare to that of compound C?
Write down only HIGHER THAN, LOWER THAN or SAME AS. (1)
3.5 Explain the answer in QUESTION 3.4 by referring to MOLECULAR STRUCTURE and TYPE of INTERMOLECULAR FORCES. (3)
3.6 Using MOLECULAR FORMULAE write down a balanced equation for the complete combustion of hexane. (3)
[14]
QUESTION 4 (Start on a new page.)
Consider the THREE incomplete organic reactions below.
- : 2-bromobutane + dilute NaOH →
- : Compound P + H2 → CH3CH(CH3)CH3
- : CH3CH2CH2COOH + CH3CH2CH2OH → Ester + Q
4.1 For reaction I write down the:
4.1.1 Type of reaction taking place (1)
4.1.2 IUPAC name of the organic product formed (2)
The dilute sodium hydroxide in reaction I is replaced with concentrated sodium hydroxide and the reaction mixture is strongly heated.
4.2 Write down a balanced equation for the reaction taking place when concentrated sodium hydroxide is used in reaction I using STRUCTURAL FORMULAE.
(Ignore the MINOR product.) (6)
4.3 For reaction II write down the:
4.3.1 Name of the type of addition reaction taking place (1)
4.3.2 Name of the catalyst used (1)
4.3.3 Structural formula for compound P (2)
4.4 Consider reaction III.
Write down:
4.4.1 The name of the type of reaction taking place (1)
4.4.2 ONE reaction condition (1)
4.4.3 Name or formula of inorganic product Q (1)
4.4.4 The structural formula and IUPAC name of the ester produced (4)
[20]
QUESTION 5 (Start on a new page.)
A factor influencing the rate of a chemical reaction is investigated by carrying out two experiments 1 and 2 in which the following reaction takes place.
CaCO3 (s) + 2 HCℓ (aq) → CaCℓ2 (aq) + H2O (ℓ) +CO2(g)
In both experiments chunks of pure calcium carbonate (CaCO3) of the same mass are added to EXCESS hydrochloric acid solution (HCℓ) in OPEN flasks. One reaction condition is changed in experiment 2.
Each flask is placed on a mass scale as shown in the diagram below.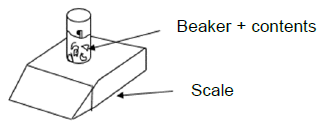
The graph below shows the changes in mass of the beaker and its contents during the reaction in experiments 1 and 2.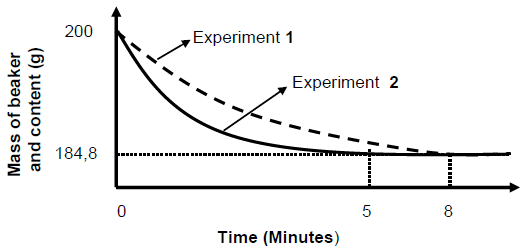
5.1 Define reaction rate in words. (2)
5.2 Write down the FORMULA or NAME of the substance responsible for
the decrease in mass of the beaker and its contents as the reaction proceeds. (1)
5.3 Write down ONE other apparatus needed to measure the rate of reaction besides the scale balance for the above experiments. (1)
5.4 Calculate the average rate of reaction in g∙min-1 for experiment 2. (3)
5.5 Calculate the mass of calcium carbonate that was used in experiment 1. (6)
5.6 The Maxwel-Boltzman distribution curves for the reaction in experiments 1 and 2 are given below.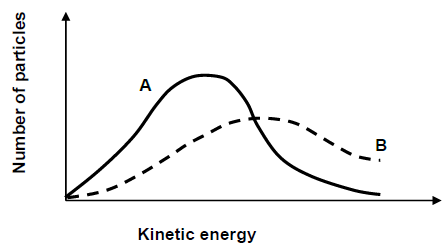
5.6.1 Which factor affecting reaction rate is investigated? (1)
5.6.2 Which curve (A or B) represents the reaction in experiment 2? (1)
5.6.3 Explain the answer to QUESTION 5.6.2 by referring to the collision theory. (3)
5.7 In a second investigation a third experiment (experiment 3), is carried out in which HCℓ of HIGHER CONCENTRATION is used. All the other conditions remain the same in experiment 3 as in experiment 1.
5.7.1 Write down an investigative question for the second investigation, in which experiment 3 is compared to experiment 1. (2)
How do the following quantities in experiment 3 compare to experiment 1? Write down LOWER THAN or HIGHER THAN or EQUAL TO.
5.7.2 Time for the reaction taken to reach completion. (1)
5.7.3 Amount of CO2 that is produced. (1)
5.8 Give a reason for the answer in QUESTION 5.7.3 above. (2)
[24]
QUESTION 6 (Start on a new page.)
Consider the reversible reaction taking place in a closed container:
H2(g) + I2(g) ⇌ 2 HI(g)
6.1 Define the term reversible reaction. (2)
The graph below shows the changes in the amount of the substances H2, I2 and HI from the moment the reactants are pumped into an empty container.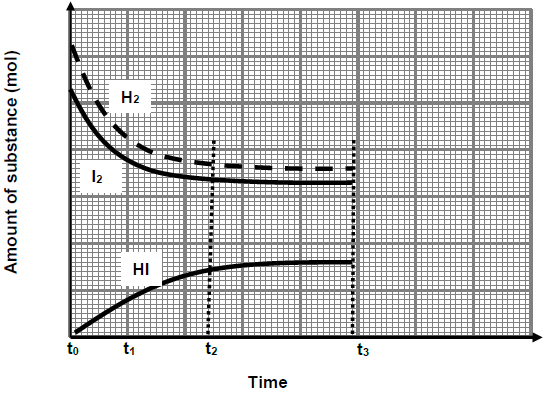
6.2 Which reaction (FORWARD or REVERSE) has a HIGHER rate of reaction during the interval t0 to t1? (1)
6.3 Did the chemical reaction stop during the interval t2 to t3? Write only YES or NO. Give a reason for the answer. (3)
At time t3 the pressure on the equilibrium system is increased by decreasing the volume at constant temperature.
6.4 How will the increase in pressure affect the following?
Write down only INCREASES, DECREASES or REMAINS THE SAME.
6.4.1 Rate of reaction. (1)
6.4.2 Number of moles of HI. (1)
6.4.3 Concentration of HI. (1)
6.5 Explain the answer to QUESTION 6.4.3 above. (2)
6.6 The table below shows the equilibrium constants, Kc values for the reaction at different temperatures.
| TEMPERATURE (°C) | Kc |
| 448 | 50,3 |
| 227 | 129 |
6.6.1 Is there a HIGH or LOW YIELD at 227 °C? Give a reason for the answer. (2)
6.6.2 Is the forward reaction EXOTHERMIC or ENDOTHERMIC? Explain the answer by referring to Le Chatelier’s principle. (4)
6.7 The reaction is started by placing hydrogen gas (H2) and iodine gas (I2) into an empty 0,5 dm3 container which is then sealed and heated.
When the reaction reaches equilibrium at 448 °C it is found that the concentration of H2 and I2 are 0,46 mol.dm-3 and 0,39 mol.dm-3 respectively.
The value of the equilibrium constant, Kc is equal to 50,3 at 448 °C.
Calculate the:
6.7.1 Concentration of HI at equilibrium (4)
6.7.2 Percentage yield at 448 °C (7)
[28]
QUESTION 7 (Start on a new page.)
7.1 Study the following reactions which show the step-by-step ionisation reaction of phosphoric acid (H3PO4).
- H3PO4 + H2O ⇌ H2PO4 + H3O+ Ka = 7,5 × 10-3
- H2PO4 + H2O ⇌ HPO42 + H3O+ Ka = 1,3 × 10-12
7.1.1 Define an acid according to Lowry-Bronsted model. (2)
7.1.2 Write down the formula of the substance that acts as an ampholyte in reaction I and II. (1)
7.1.3 Give a reason for the answer in QUESTION 7.1.2 above. (2)
7.1.4 Which substance, H2PO4 or HPO42 , will have a HIGHER Kb value? Give a reason for your answer. (3)
7.2 Consider the hydrolysis of the ion C2HO4 represented by the balanced equation below.
C2HO4 (aq) + H2O(ℓ) ⇌ C2H2O4 + OH
7.2.1 Define the term hydrolysis. (2)
7.2.2 Write down the conjugate base of C2H2O4. (2)
7.2.3 Give a reason by referring to substance(s) in the equation why the hydrolysis of C2HO4 produces an ALKALINE solution? (2)
7.3 A group of learners perform a titration to determine x number of moles of water of crystallisation in hydrated oxalic acid (C2O4H2 ∙x H2O).
They first prepared a solution of hydrated oxalic acid, by adding 7,56 grams of hydrated oxalic acid to water and made a volume of 250 cm3 solution.
During a titration 25 cm3 of the solution of hydrated oxalic acid is neutralised by exactly 24 cm3 of a 0,5 mol∙dm-3 solution of sodium hydroxide according to the balanced equation:
C2O4H2. x H2O(s) + 2 NaOH(aq) → C2O4Na2(aq) + (x+2) H2O(ℓ)
(Water of crystallisation does not react with the base.)
7.3.1 Define a strong base. (2)
7.3.2 Calculate the pH of the 0,5 mol∙dm-3 sodium hydroxide solution. (5)
7.3.3 Determine the value of x by calculation. (7)
[28]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
Physical Science Paper 1 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate space on the ANSWER BOOK.
- Answer ALL the questions.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Number the answers correctly according to the numbering system used in this question paper.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Give brief motivations, discussions, et cetera where required.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Start EACH question on a NEW page.
- All diagrams are not necessarily drawn according to scale.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four possible options are provided as answers to the following questions. Choose the answer and write only the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 Vuyo, the driver of a minibus that is moving at a constant velocity, on a horizontal road, sees that the sliding door of the minibus is open. He suddenly applies his brakes and the door closes. Which ONE of the following Physics laws or principles best explains why Vuyo could close the door?
- Law of conservation of mechanical energy
- Principle of conservation of linear momentum
- Newton’s First Law of Motion
- Newton’s Second Law of Motion (2)
1.2 A 2 kg mass piece is placed on a 2 kg trolley, which is at rest on a frictionless horizontal surface. When a force F is applied on the trolley, it accelerates with an acceleration of a as shown in FIGURE 1. A 1 kg mass piece is then placed on the trolley with the 2 kg mass piece and the same force F is applied on the trolley as shown in FIGURE 2.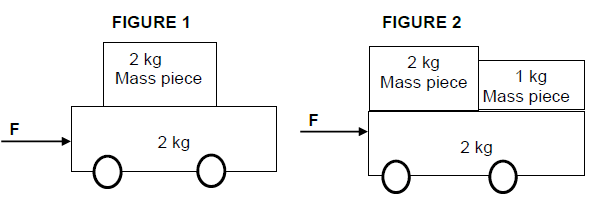
What will be the acceleration of the trolley in FIGURE 2?
- 1 a
4 - 2 a
3 - 4 a
5 - 5 a (2)
4
1.3 Two cars are involved in an INELASTIC head-on collision. Which ONE of the following combinations correctly describes the kinetic energy and the momentum of the system?
| TOTAL KINETIC ENERGY | TOTAL MOMENTUM | |
| A | Not conserved | Conserved |
| B | Conserved | Not conserved |
| C | Conserved | Conserved |
| D | Not conserved | Not conserved |
(2)
1.4 The graph below shows the relationship between the power delivered by the engine of a car and the average speed of the car.
The gradient of the above graph represents the …
- momentum of the car.
- work done by the engine of the car.
- kinetic energy of the car.
- applied force of the engine on the car. (2)
1.5 A ball is dropped from a certain height. Which ONE of the position versus time graphs below best represents the motion?
(2)
1.6 … is the main principle applied when measuring the rate of blood flow or the heartbeat of a fetus in the womb.
- Photoelectric effect
- Doppler effect
- Huygen’s principle
- Diffraction (2)
1.7 The electrostatic force between two point charges that are placed a distance r apart in a vacuum is F.
The distance between the charges is changed and the electrostatic force between them changes to 16 F. What is the new distance between the point charges in terms of r?
- 8 r
- 1 r
8 - 4 r
- 1 r (2)
4
1.8 Three identical resistors are connected to a battery as shown on the circuit diagram below. The resistance of the connecting wires can be ignored.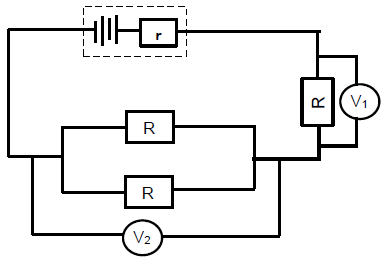
Which ONE of the following best represents the relationship between the readings of the voltmeters?
- V1 = ½ V2
- V1 = 2 V2
- V1 = V2
- V1 = ⅓ V2 (2)
1.9 The maximum emf of an AC generator can be increased by ...
- changing the polarity of the magnets.
- using larger slip rings.
- using larger brushes.
- increasing the number of turns on the coil. (2)
1.10 In a house, appliances are usually connected in parallel to each other because …
- the resistance of each resistor will decrease.
- each appliance can operate independently.
- the current supply to each appliance will be higher.
- each appliance will have maximum voltage.
Which ONE of the following combinations about reasons for connecting appliances in parallel in a house is correct?
- (i) and (ii) only
- (i), (ii) and (iii) only
- (ii), (iii) and (iv) only
- (i), (ii), (iii) and (iv) (2)
[20]
QUESTION 2
A block of mass 22 kg is placed on a rough inclined surface and is connected to a 15 kg block which hangs vertically by means of a light inextensible string passing over a frictionless pulley, as shown in the diagram below. When the inclined surface is at an angle ? to the horizontal, the 15 kg block moves downwards (as shown by the arrow alongside the diagram) at a CONSTANT VELOCITY.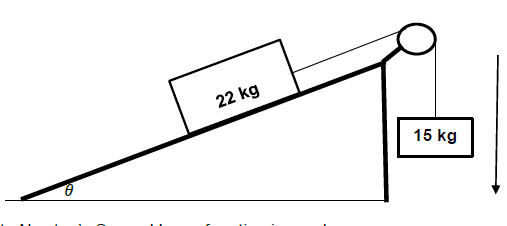
2.1 State Newton’s Second Law of motion in words. (2)
2.2 Draw a labelled free-body diagram of all forces acting on the 22 kg block. (4)
2.3 A constant frictional force of 43,86 N acts on the 22 kg block. Calculate the magnitude of the angle ? that will keep the system moving at a constant velocity.
(4)
2.4 A force of 172 N is now applied on the 22 kg block and the system of blocks accelerates down the slope. The kinetic frictional force acting on the 22 kg block while it accelerates downward remains the same.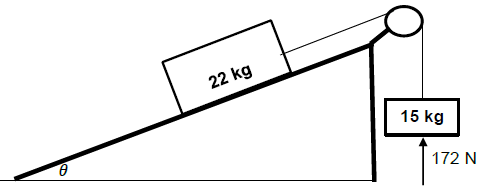
2.4.1 Explain why the kinetic frictional force remains the same even though the system accelerates. (2)
2.4.2 Calculate the acceleration of the system of blocks. (6)
[18]
QUESTION 3
A ball of mass 500 g is thrown vertically downwards with a velocity of 3,28 m.s-1 from the top of a 30 m high building, point A. After 1,2 s the ball passes the top edge of a window and goes on to hit the ground below. The ground exerts a force of 205 N on the ball and it bounces off to a maximum height ∆y, which is at the same level as the lower edge of the window, as shown in the diagram below. The ball is in contact with the ground for 0,1 s. Ignore the effects of air resistance.
Calculate the:
3.1 Velocity of the ball after 1,2 seconds (3)
3.2 Displacement of the ball after 1,2 s of its motion (4)
3.3 Magnitude of the velocity with which the ball hits the ground (3)
3.4 Velocity with which the ball bounced off the ground (4)
3.5 Length of the window (5)
[19]
QUESTION 4
During a demonstration to determine the recoil velocity of a certain type of gun, a bullet of mass 5 g is fired from a gun of mass 5 kg. The bullet moves horizontally at a constant velocity into a wooden block of mass 2 kg that hangs vertically from a ceiling by means of a light inextensible string. The block-bullet system swings to a vertical height of 6 cm above its original position as shown in the diagram below. Ignore the effects of air resistance.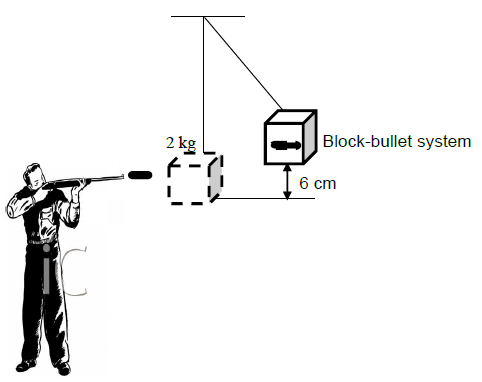
4.1 State the principle of conservation of linear momentum in words. (2)
4.2 Suppose that the original position of the wooden block is zero position. Calculate the:
4.2.1 Velocity of the bullet before it entered the wooden block (5)
4.2.2 Recoil velocity of the gun (3)
[10]
QUESTION 5
A skateboarder skates from point A of the track, which is at a vertical height of 15 m, and aims to come to rest at point D on the other side as shown on the diagram below. Section AB is frictionless while BC and CD are rough. The combined mass of the skateboarder and the skateboard is 55 kg. The skateboarder experiences a frictional force of 15 N while moving from point C to point D. Ignore the effects of air resistance.
5.1 State the principle of conservation of mechanical energy in words. (2)
5.2 Calculate the kinetic energy of the skateboarder at point B. (3)
5.3 State the work-energy theorem in words. (2)
5.4 Use energy principles to calculate the:
5.4.1 Kinetic energy of the skateboarder at point C (4)
5.4.2 Magnitude of the frictional force along BC that will enable the skateboarder to come to rest at point D (5)
[16]
QUESTION 6
A laboratory assistant conducted an experiment to determine the frequency of a tuning fork. The tuning fork is set on vibration and mounted on a stand as shown in the inserted sketch. He then moves towards and away from the tuning fork with a detector which records the frequency of sound emitted by the tuning fork. The graph below represents the results he recorded. Take velocity of sound in air as 340 m.s-1.
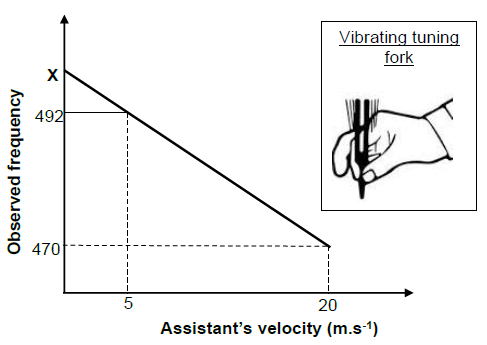
6.1 Name the phenomenon represented on the graph. (1)
6.2 Does the graph represent the instance when the laboratory assistant was moving TOWARDS or AWAY from the tuning fork? Explain your answer. (2)
6.3 Write down the relationship between the laboratory assistant’s velocity and the observed frequency. (2)
6.4 What does X, the intercept on the vertical axis, represent? (2)
6.5 Calculate the frequency of the tuning fork. (5)
6.6 The laboratory assistant intends to use the tuning fork to produce oscillations with wavelength of 0,71 m for a Grade 10 demonstration lesson. Use a calculation to determine whether or not the tuning fork will be suitable for the demonstration. (3)
[15]
QUESTION 7
A small, charged sphere X carrying a charge of +2 x 10-6 C is placed in a vacuum. When another small sphere Y of mass 5 mg carrying a negative charge is placed at point P, it accelerates from rest to a velocity of 6,25 x 103 m.s-1 just before sphere Y touches sphere X. The time taken for sphere Y to reach sphere X is 2 x 10-3 seconds.
7.1 Calculate the number of electrons that were removed from the sphere X to obtain the charge on the sphere. (3)
7.2 Draw the electric field pattern around the charges while sphere Y is still at point P. (3)
7.3 Calculate the:
7.3.1 Net electric field strength at point P due to the charged sphere X (6)
7.3.2 Distance between the charges before sphere Y moved (3)
7.3.3 Charge on sphere Y (4)
[19]
QUESTION 8
In an experiment to determine the emf (ε) and internal resistance r, of a battery, a group of learners connected the circuit diagram below. They recorded the ammeter and voltmeter readings. They repeated the experiment each time with a different resistance.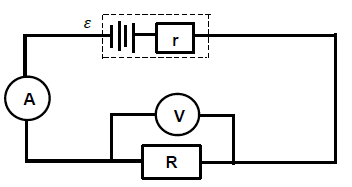
Their results are plotted in the graph below.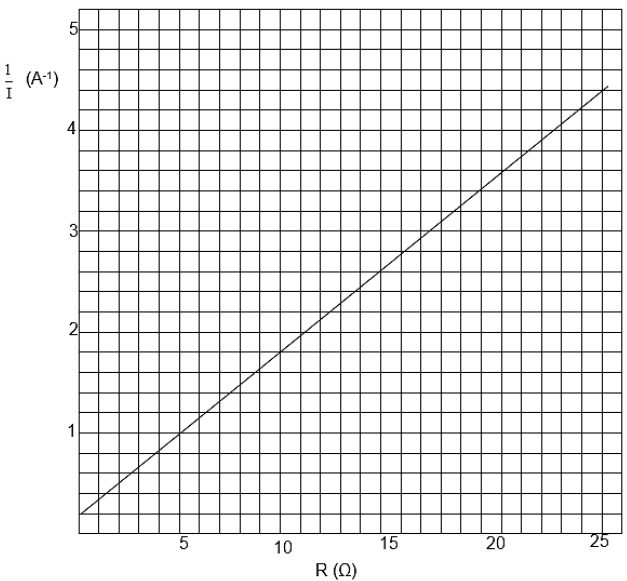
8.1 What is the independent variable for this experiment? (1)
8.2 Using the equation 1 = R + r calculate the:
I emf
8.2.1 Emf ? of the battery (4)
8.2.2 Internal resistance of the battery (3)
8.3 In the circuit diagram below the battery used in the above experiment is connected to three resistors and a toy car that is rated at 3 W, 1,5 V.
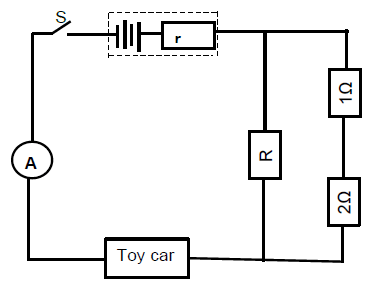
When the switch S is closed, calculate the:
8.3.1 Reading on the ammeter (3)
8.3.2 Resistance of resistor R (6)
8.4 If the resistor R is removed from the circuit, will the toy car be able to operate as rated? Answer only YES or NO. Explain your answer. (3)
[20]
QUESTION 9
A learner built an electrical device for her Eskom Expo science project as shown below.
9.1 What is name of the electrical device represented above? (1)
9.2 State the energy conversion taking place in this device. (2)
9.3 If current flows in the device as indicated by the arrows in the diagram above, in which direction will section AB rotate? Answer CLOCKWISE or ANTI- CLOCKWISE. (1)
9.4 Her teacher advised her to modify the above device into one that can supply households with electricity. How can she modify the device for the desired purpose? (2)
9.5 A kettle rated at 1 500 W is plugged into a wall socket that supplies 230 V. Calculate the:
9.5.1 Resistance of the conducting coil in the kettle (3)
9.5.2 Maximum (peak) current that passes through the kettle (4)
[13]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Acceleration due to gravity | g | 9,8 m•s-2 |
Universal gravitational constant | G | 6,67 × 10-11 N•m2•kg-2 |
Speed of light in a vacuum | c | 3,0 × 108 m•s-1 |
Planck's constant | h | 6,63 × 10-34 J•s |
Coulomb's constant | k | 9,0 × 109 N•m2•C-2 |
Charge on electron | e | -1,6 × 10-19 C |
Electron mass | me | 9,11 × 10-31 kg |
Mass of earth | M | 5,98 × 1024 kg |
Radius of earth | RE | 6,38 × 103 km |
TABLE 2: FORMULAE
MOTION
| vf = vi + aΔt | Δx = ViΔt + ½aΔt2 or Δy = ViΔt2 + ½aΔt2 |
Vf2 = Vi2 + 2aΔx or Vf2 = vi2 + 2aΔy | Δx = [Vi + Vf]Δt or Δy = [Vi + Vf]Δt |
FORCE
Fnet = ma | p= mv |
fsmax = µsN | fk = µkN |
FnetΔt = Δp | w =mg |
F = Gm1m2 | g = G M |
WORK, ENERGY AND POWER
W =FΔxcosθ | U= mgh or EP = mgh |
K = ½mv2 or Ek = ½mv2 | Wnet = ΔK or Wnet = ΔEk ΔK = Kf −Ki or ΔEk =Ekf − Eki |
Wnc= ΔK + ΔU or Wnc= ΔEk + ΔEp | P = W Δt |
Pav = Fv |
WAVES, SOUND AND LIGHT
v = f λ | T =1/f |
fl = v ± vl fs fl = v ± vl fb | E = hf or E = h c |
E = W0 + Ek where | |
ELECTROSTATICS
| F = kQ1Q2 r2 | E = KQ |
E = V | E = F |
V = W | n = Q |
ELECTRIC CIRCUITS
R = V | emf (ε) = I(R + r) |
RS = R1 + R2 + ....... | q = I Δt |
W = Vq | P= W |
ALTERNATING CURRENT
I rms = Imax | Paverage = VrmsIrms |
Mechanical Technology: Automotive Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
MEMORANDUM
QUESTION 1: MULTIPLE-CHOICE QUESTIONS (GENERIC)
1.1 C (1)
1.2 B (1)
1.3 C (1)
1.4 A (1)
1.5 B (1)
1.6 C (1) [6]
QUESTION 2: SAFETY (GENERIC)
2.1 Gas welding (PPE)
- Eye protection
- Overall / leather apron
- Safety boots
- Gloves (Any 2 x 1) (2)
2.2 Safety rules that must be followed whilst the surface grinder is in operation:
- Make sure that the sparks are of no danger to co-workers.
- Do not force the material onto the grinding wheel.
- Do not plunge grind.
- Bring the material slowly into contact with the grinding wheel.
- Never clean or adjust the machine whilst it is in motion.
- Use cutting fluid.
- Know where the emergency stop is located.
- Stop the machine before any adjustment.
- Keep tools clear from moving parts.
(Any 2 x 1) (2)
2.3Completing a task on any machine:
- Switch the machine off. (1)
2.4 TWO safety measures to observe before switching the angle grinder on:
- Make sure that there are no cracks or chips on the disc.
- Make sure that the emery disc that is fitted is rated above the revolutions at which it is turned by the motor.
- Make sure that the space between the tool rest and the emery disc does not exceed 3 mm.
- Ensure that guards are in place.
- Do not stand in front of the machine when switching it on; wait until it reaches its full speed.
- Do not force or bump the work piece against the emery disc.
- Grind only on the front surface of the wheel, not the sides.
- All grinding machines must have a sign indicating the revolutions at which the spindle rotates. (Any 2 x 1) (2)
2.5 Importance of a welding helmet:
- To protect your eyes and face from ultra-violet rays and radiation (1)
2.6 Types of workshop layouts:
- Process layout
- Product layout (2)
[10]
QUESTION 3: MATERIALS (GENERIC)
3.1
| MATERIALS | DIFFERENT TYPES OF TESTS | ||
| Sound | Filing | Bend | |
| Cast iron | Very dull sound | Easy | Cannot bend / Snaps/breaks/ Fracturea easily |
| Mild steel | Medium metallic sound | Easy | Benda easily |
(6)
3.2 Heat treatment process:
- Is the heating and cooling of metals in their solid state so as to change their properties(1)
3.3 Hardness factors:
- Workpiece size
- Quenching rate
- Carbon content (Any 2 x 1) (2)
3.4 Heat treatment processes:
3.4.1 Tempering
- Is a process applied to steel and it relieves the strains induced during the hardening process.
- It decreases the degree of hardness
- It increases toughness
- It reduces brittleness
- It gives steel fine grain structure (Any 2 x 1) (2)
3.4.2 Annealing
- Relieves internal stress
- Softens the metal
- Makes metal ductile
- Refines the grain structure
- Reduces brittleness (Any 2 x 1) (2)
3.5 Hardness of steel depends upon
- Carbon content (1)
[14]
QUESTION 4: MULTIPLE-CHOICE QUESTIONS (SPECIFIC)
4.1 A (1)
4.2 C (1)
4.3 D (1)
4.4 B (1)
4.5 B (1)
4.6 D (1)
4.7 C (1)
4.8 D (1)
4.9 B (1)
4.10 A (1)
4.11 C (1)
4.12 D (1)
4.13 B (1)
4.14 A (1) [14]
QUESTION 5: TOOLS AND EQUIPMENT (SPECIFIC)
5.1 Bubble gauge:
5.1.1 Bubble gauge (1)
5.1.2
- A – King-pin inclination scale
- B – Caster scale
- C – Camber scale
- D – Gauge zero scale
- E – Mounting equipment on wheel (5)
5.1.3
- Caster angle
- Camber angle
- King-pin inclination (3)
5.2 Set-up procedure to read camber:
- Ensure that the wheels are in a straight ahead position.
- Mount the bubble gauge on the centre of the wheel.
- Zero the bubble gauge on the gauge zero scale.
- Take the reading on the camber scale.
- Do the same for the other wheels. (5)
5.3 Dynamic balancing of wheels:
- The plane of imbalance
- The extent of unbalanced forces
- The sense of direction of these forces (clockwise or counter clockwise / anticlockwise)
- Run-out of the tyre and wheel assembly (Any 3 x 1) (3)
5.4 Tools:
5.4.1 Turn table:
- To turn the front wheel 20° in and zero the bubble gauge and then turn the wheel 20° out to check the castor reading (2)
5.4.2 Wheel balancer:
- To balance the wheels of a vehicle for static and dynamic balance. (2)
5.4.3 Optical alignment tool:
- To check the toe-in and toe-out of a vehicle (2)
[23]
QUESTION 6: ENGINES (SPECIFIC)
6.1 Causes of vibration:
- The action of unbalance forces upon the shaft
- The twisting effects of the power stroke (2)
6.2 Types of vibration damper:
- Friction face-type
- Combined rubber and friction disc (2)
6.3 In-built engine balance features:
6.3.1 Crankshafts are carefully balanced with webs extended and drilled to form balance mass piece at points opposite the connecting rods. (2)
6.3.2 Connecting rods and pistons are kept as light as possible to reduce reciprocating forces. (2)
6.3.3 Flywheels are carefully balanced and are usually fitted to the crankshaft flange in one position only. (2)
6.4 Factors that determine engine configuration:
- Number of cylinders
- Position of cylinders
- Engine layout
- Firing order
- Engine location and mounting (Any 3 x 1) (3)
6.5 Types of engine configuration:
- In-line engine
- V-type engine
- Horizontally opposed engine (Any 2 x 1) (2)
6.6 Identification of an engine configuration:
- The crankshaft of a V-engine (1)
6.7 Factors that determine the firing order:
- The position of the crank on the crankshaft
- The arrangement of the cams on the camshaft (2)
6.8 Firing order of a 5-cylinder in-line engine:
- 12453 OR 13542 (1)
6.9 Turbocharger internal components:
- A – Turbine exhaust gas outlet
- B – Turbine wheel OR impeller
- C – Turbine exhaust gas inlet
- D – Compressor air discharge
- E – Compressor
- F – Compressor air inlet (6)
6.10 Disadvantages of a turbocharger:
- It can have lag problems.
- It tends to heat up the air, reducing density.
- Some require shut-down process.
- It requires pressure lubrication for high speed bearings.
- Its lubricant must be air cooled.
- Over-revving must be controlled by waste gate.
(Any 3 x 1) (3)
[28]
QUESTION 7: FORCES (SPECIFIC)
7.1 Compression ratio:
- The compression ratio of an internal combustion engine is the ratio of compression of the inlet charge during the compression stroke to the total volume of the cylinder. (2)
7.2 Compression ratio: Swept volume:
- ????? ?????? = ∏?2 ?
4
= ∏(9.02) × 11
4
= 700 cm3
Compression ratio = ??+??
??
= 700+70
70
11 : 1 (6)
7.3 New compression ratio:
- Swept volume = ∏(9,612) × 11
4
= 797,865 cm3
Compression ration = 797,865 + 70
70
=12,4 : 1 (4)
7.4 Methods used in raising compression ratio:
- Remove shims between the cylinder block and cylinder head.
- Fit thinner cylinder head gasket.
- Machine metal from cylinder head.
- Skim metal from cylinder block.
- Fit piston with higher crown.
- Fit crankshaft with longer stroke.
- Increase the bore of the cylinders. ( Any 4 x 1) (4)
7.5Indicated power:
- Indicated power is a measure to determine the total power developed by the burning of fuel in the combustion chamber of an internal combustion engine. (2)
7.6 Power calculations:
7.6.1 Indicated power
- = P × L × A × N × n
P = 1200000 Pa
L= 86
1000
=0,086 m
A = π?2
4
= π× 0,092
4
=6,36 × 10-3 m2
N = 4200
60×2
= 35 r/s
N = 4 cylinders
Indicated power = 1200000 × 0,086 × 6,36 × 10-3 × 35 × 4
= 91889,28 W
= 92 kW (7)
7.6.2
- Brake power = 2 π NT
N =4200
60
70 r/s
= 2 × π × 70 × 180
=211115,03 W
=79168,13 W
=79,2 kW (3)
7.6.3 Mechanical efficiency
- = ?? ×100%
??
= 79,2 ×100%
92
= 82,5% (2)
7.7 Term definition:
- It is the percentage energy that an engine puts out due to mechanical losses as compared to the ideal engine power. (2)
[32]
QUESTION 8: MAINTENANCE (SPECIFIC)
8.1 Reasons for high CO (carbon monoxide) reading:
8.1.1
- Too rich mixture
- Ignition misfire
- Dirty or restricted air filter
- Improper operation of the fuel delivery system
- Faulty thermostat or coolant sensor
- Non-functioning PVC valve system
- Catalytic converter not working (Any 3x1) (3)
8.1.2 Corrective measures:
- Reset fuel mixtures.
- Check for misfire and repair.
- Replace air filter.
- Check and correct fuel delivery system. (Any 3 x 1) (3)
8.1.3 Gases analysed:
- CO2
- SO2
- NO
- HC
- O2 (Any 3 x 1) (3)
8.2 Cylinder leakage testing:
- Wet test (1)
8.3 Cylinder leakage and causes:
8.3.1 Leakage inlet valve (1)
8.3.2 Blown cylinder head gasket or cracked cylinder block (1)
8.3.3 Piston rings are worn (1)
8.4 Oil pressure testing:
- Oil pressure when the engine is idling.
- Oil pressure when the engine is cold.
- Oil pressure when the engine is hot.
- Oil pressure on high revolution. (4)
8.5 Causes of low fuel pressure reading:
- Faulty fuel pump
- Blocked or restricted fuel filter
- Cracked or restricted fuel line
- Clogged pump inlet strainer
- Low voltage to fuel pump
- Faulty fuel pressure regulator
- Faulty fuel pump relay
- Empty fuel tank (Any 3 x 1 ) (3)
8.6 Cooling system pressure testing:
- Water hoses
- Water pump
- Radiator
- Corroded core plugs
- Interior heater radiator
- Faulty radiator cap (Any 3 x 1 ) (3)
[23]
QUESTION 9: SYSTEMS AND CONTROL (AUTOMATIC GEARBOX) (SPECIFIC)
9.1 Purpose of automatic gearbox:
- To relieve the driver of clutch and gearshift operation, thereby allowing the driver to concentrate on driving the vehicle (2)
9.2 Advantages of an automatic gearbox
- It reduces driving fatigue.
- It ensures reduction of wheel spin under bad road condition.
- The vehicle can be stopped suddenly without the engine stalling
- The system puts a damper on/muffles all engine vibrations.
(Any 3 x 1) (3)
9.3 Torque converter:
9.3.1 Torque converter (1)
9.3.2 Parts
- A – One-way clutch
- B – Turbine
- C – Pump
- D – Turbine shaft
- E – Gearbox housing (5)
9.3.3 Torque converter functions:
- Transfers engine torque to the transmission.
- Multiplies the engine torque.
- Provides a direct drive from engine to transmission.
- It muffles/puts a damper on all engine vibrations.
- It acts as flywheel. (Any 3 x1 ) (3)
9.3.4 Function of parts:
- It sets the fluid in motion at high pressure to the turbine, thereby causing the turbine to rotate with great torque. (2)
9.4 Torque multiplication:
- As the car speed increases, the torque multiplication tapers off gradually. (2)
[18]
QUESTION 10: SYSTEMS AND CONTROL (AXLES, STEERING GEOMETRY AND ELECTRONICS) (SPECIFIC)
10.1 Properties of a good steering mechanism:
- Light and easy to control
- Free from vibration and road shocks
- Self-centring
- Able to operate effectively under the influence of the suspension and braking system
- It must be as direct as possible to reduce too much driver’s attention.
(Any 4 x 1) (4)
10.2 (4)
(4)
10.3 Alignments:
10.3.1 Caster angle:
- It gives self-centring action to the steering thereby keeping the wheels in straight ahead position.(2)
10.3.2 Ackermann principle:
- To avoid the need for tyres to slip sideways when following the path around a curve. (2)
10.3.3 King pin inclination:
- To bring the front wheel back to the straight ahead position after rounding a corner without any driver effort. (2)
10.4 Camber:
10.4.1 Positive camber. (2)
10.4.2
- A – Tyre
- B – Vertical line
- C – Centre line
- D – Positive camber angle
- E – Lower control arm
- F – Road surface (6)
10.4.3 Positive camber angle is the outward tilt of a front wheel away from The vehicle when viewed from the front. (1)
10.5 Factors to be taken into account before attempting alignment adjustment:
- Kerb mass must be checked against the manufacturer’s specifications
- Uneven wear on tyres
- Tyre pressure
- Run-out on wheels
- Kingpins and bushes
- Suspension ball joints for wears
- Suspension bushes for excessive free movements
- Tie-rod ends
- Sagged springs
- Ineffective shock absorbers
- Spring U-bolts
- Chassis for possible cracks
- Wheel must be balanced
- Wheel alignment specifications
- Drive shaft CV-joints (Any 5 x 1) (5)
10.6 Purpose of wheel balancing:
- To avoid shimming and bouncing of wheel assembly which can cause wearing of the steering mechanism and suspension parts. (2)
10.7 Wheel balancing:
- Static balance
- Dynamic balance (2)
[32]
TOTAL: 200