Adele
RATE AND EXTENT OF REACTIONS QUESTIONS AND ANSWERS GRADE 12
Activity 1
Consider the reaction
H2 +I2→2HI (∆H < 0)
1. Is this reaction exothermic or endothermic? (1)
2. State a reason for your answer in 1.1. (1)
3. How does energy of the products compare to that of the reactants? (2) [4]
Solutions
1. Exothermic (reaction). ✓ (1)
2. ∆H<0✓ (1)
3. The energy of the products is less than ✓ that of the reactants. ✓ (2) [4]
Activity 2
1. Hydrogen gas may be prepared by the reaction of zinc metal with dilute hydrochloric acid. The chemical equation for this reaction is :
Zn(s) + 2HCℓ(aq) → ZnCℓ2(aq) + H2(g) ∆H < 0
A learner determined the volume of the hydrogen produced with time at two temperatures and two grades of Zn; powder and solid pellets. He used the same mass of zinc and the same volume and concentration of hydrochloric acid for each experiment and plotted the following graphs.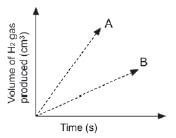
Which combination of temperature and surface area will be represented by A? (2)
Temperature | State of Zn | |
| A | High | Pellets |
| B | High | Powder |
| C | Low | Pellets |
| D | Low | Powder |
2, The graphs below represent the molecular distribution for a reaction at different temperatures.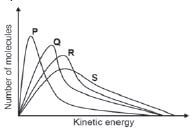
Which ONE of the graphs above represents the reaction at the highest temperature? (2)
- P
- Q
- R
- S [4]
Solutions
1. B 33 (2)
2. D 33 (2) [4]
Activity 3
Give ONE term for each of the following descriptions by choosing a term from the list above. Write down only the term next to the question number.
| Surface Area; Catalyst; Elastic collision; Effective collision; Activated complex; Concentration; Temperature; Heat of reaction; Activation energy. |
1. A chemical substance that speeds up the rate of a chemical reaction by lowering the net activation energy. (1)
2. A collision in which the reacting particles have sufficient kinetic energy and correct orientation. (1)
3. The factor responsible for increasing the rate of a reaction when a solid is broken up into smaller pieces. (1)
4. The temporary unstable state that is formed during the course of a chemical reaction. (1)
5. A measure of the average kinetic energy of the particles in a gas. (1)
6. The net amount of energy released or absorbed during a chemical reaction. (1) [6]
Solutions
1. Catalyst. ✓ (1)
2. Effective collision. ✓ (1)
3. Surface Area. ✓ (1)
4. Activated complex. ✓ (1)
5. Temperature. ✓ (1)
6. Heat of reaction. ✓ (1) [6]
e.g. Worked example
Learners use hydrochloric acid and a sodium thiosulphate (Na2S2O3) solution to investigate the relationship between rate of reaction and temperature. The reaction that takes place is represented by the following equation:
Na2S2O3(aq) + 2HCℓ(aq) → 2NaCℓ(aq) + S(s) + H2O(ℓ) + SO2(g)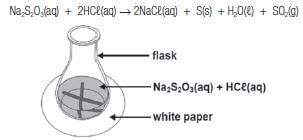
They add 5 cm3 2 mol·dm–3 hydrochloric acid solution to 50 cm3 sodium thiosulphate solution in a flask placed over a cross drawn on a sheet of a white paper, as shown in the diagram below. The temperature of the mixture is 30°.
They measure the time it takes for the cross to become invisible. The experiment is repeated with the temperature of the mixture at 40˚C, 50˚C and 60˚C.
- Write down:
1.1 The possible hypothesis for this investigation.
1.2 NAME or FORMULA of the product that requires the need to work in a well-ventilated room.
1.3 NAME or FORMULA of the product that causes the cross to become invisible. - Apart from the volume of the reactants, state ONE other variable that must be kept constant during this investigation.
- Why is it advisable that the same learner observes the time that it takes for the cross to become invisible?
- What experimental technique was used to measure the rate of reaction in this investigation?
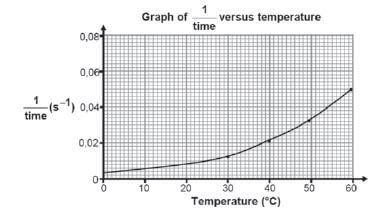
- The shown graph below is obtained from the results.

5.1 What is the relationship represented by 1/time on the vertical axis?
5.2 What conclusion can be drawn from the results obtained?
The learners collected washed and dried sulphur. They weighed the dry sulphur and its mass was 0,12g. Calculate the percentage yield of sulphur.
Solutions
- 1.1 E.g. The rate of reaction increase with increase in temperature.
1.2 Sulphur dioxide/SO2.
1.3 Sulphur/S. - Concentration of reactants (HCℓ and Na2S2O3).
- Different people have different sight abilities/reaction times. (To ensure that the results are reliable).
- Turbidity.
- 5.1 Reaction rate.
5.2 The reaction rate increases with increase in temperature. - Na2S2O3(aq) + 2HCℓ(aq) → 2NaCℓ(aq) + S(s) + H2O(ℓ) + SO2(g)
n(HCℓ) = CV
n(HCℓ) = (2)(0,005)
n(HCℓ) = 0,01 mol
2 n(HCℓ) = 1 n(S)
n(S) = 0, 005 mol
n = m/M
0,005 = m/32
m(s) = 0,16 g
Percentage Yield = 0,12/0,16 × 100
Percentage Yield = 75 (%)
Activity 4
81,1 g of nicotine consists of 60,07 g of carbon, 14,01 g of nitrogen and 7,02 g of hydrogen.
Determine the:
- The empirical formula of nicotine. (13)
- Molecular formula of nicotine if its molar mass is 162,26g. (4) [17]
Solutions
Determining the percentage composition by molar mass of each element:
- C = 60,07 × 100 = 74,04%
81,1 - N = 14,01 × 100 = 17,27%
81,1
H =7,02 × 100 = 8,65%
81,1Element g per 100g n = m/M
for 100gn = m/M
for 81,1gSimplest Ratio C 74,04 n = 74,04
12
= 6,1760,07 = 5
125 N 17,28 n = 17,27
14
=1,2314,01 = 1
141 H 8,65 n = 8,65
1
= 8,657,02 = 7
17
Empirical formula of nicotine is C5NH7 (13)
2. Now calculate the molar mass of the substance using empirical formula
- M(C5NH7) = 81 g/mol
x(81) = 162,2
x = 2 (meaning every atom in the empirical formula must be multiplied by 2)
Molecular formula of nicotine is C10N2H14 (4) [17]
CHEMICAL EQUILIBRIUM QUESTIONS AND ANSWERS GRADE 12
Activity 1
Write the name of a reaction in which all reactants and products are in the same phase (1) [1]
Solution
- Homogeneous ✓ [1]
Activity 2
Consider the following reversible reaction that is in equilibrium in a closed system (the symbols represent chemicals which are unnamed; if you see symbols like X or Y which aren’t on the Periodic Table, it often means you’ve got to work out what kinds of substances they are).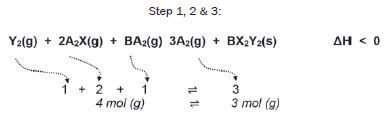
Step 4/5:
∆H < 0
- the forward reaction is exothermic
- the reverse reaction is endothermic
In each of the following cases:
- Explain how the equilibrium will be affected (which reaction will be favoured)
- State how the concentration of the product A2(g) will change.
CASE 1 The reaction system is heated. (3) [3]
Solution
Case 1.
If the reaction system is heated, the temperature of the system increases.
According to Le Châtelier’s Principle, increasing the temperature of the reaction system (i.e. heating it) favours the reaction that will decrease the temperature of the system
- ∴favours the endothermic ✓ reaction but ∆H < 0
- ∴the reverse reaction is endothermic
- ∴the reverse reaction is favoured✓∴the 3A2(g) + BX2Y2(s) reacts faster than it is produced
- ∴the concentration of the A2(g) decreases. ✓
(Remember: The opposite is true if the reaction vessel is cooled.) [3]
hint
You must be able to use Le Châtelier’s Principle to identify the factor that will increase the yield of the products and what influence that factor will have on the reaction AT EQUILIBRIUM
CASE 2 Y2(g) is added to the vessel at constant pressure and temperature (3) [3]
Solution
Case 2.
Adding Y2(g) increases the concentration of the Y2(g). ✓
According to Le Châtelier’s Principle, increasing the concentrationof Y2(g) favours the reaction that decreases the concentration of Y2(g)
- ∴the reaction that uses of Y2(g) as a reactant
- the forward reaction is favoured. ✓
- ∴the rate at which A2(g) and BX2Y2(s) is produced, increases
- the concentration of the A2(g) increases. ✓ [3]
CASE 3 A2(g) is removed from the vessel at constant pressure and temperature. (3) [3]
Solution
Case 3.
Removing A2(g) decreases the concentration of the A2(g). ✓
According to Le Châtelier’s Principle, decreasing theconcentration of the A2(g) favours the reaction that increases the concentration of the A2(g);
- the reaction that makes A2(g) as a product
∴ the forward reaction is favoured. ✓
the rate at which A2(g) and BX2Y2(s) is produced, increases
the concentration of the A2(g) increases. ✓ [3]
CASE 4 Some of the BX2Y2(s) is removed from the system at constant pressure and temperature. (3) [3]
Solution
Case 4.
Removing BX2Y2(s) has no effect on the equilibrium as it is a solid. ✓
Removing a solid from an equilibrium system does not disturb the concentration. ✓ (Solids in equilibrium reactions have very little change in mass, hence they are ignored)
The concentration of A2(g) remain the same. ✓ [3]
CASE 5 The pressure on the system is increased (or the volume is decreased) (3) [3]
Solution
Case 5.
According to Le Châtelier’s Principle, increasing the pressure (or decreasing the volume) of a gaseous system favours the reaction that decreases the pressure of the system by decreasing the total number of gaseous moles in the system. ✓
- the forward reaction is favoured. ✓
- the rate at which A2(g) and BX2Y2(s) is produced, increases
- the concentration of the A2(g) increases. ✓ [3]
hint
There are 4 gaseous moles on the left, and 3 gaseous moles on the right.
Activity 3
Say whether the statement is TRUE or FALSE: If the equilibrium constant for the reaction A2(g) + B2(g) ⇌ 2AB(g) is equal to K, then the equilibrium constant for the reverse reaction
2AB(g) ⇌ A2(g) + B2(g) is also equal to K. (3) [3]
Solution
FALSE✓✓ . ... for the reverse reaction 2AB(g) ⇌ A2(g) + B2(g) is equal to 1/K ✓✓ OR ... less (smaller) than K [3]
Activity 4
The industrial preparation of hydrogen gas is represented by the equation below:
CH4(g) + H2O(g) ⇌ CO(g) + 3H2(g) ∆H > 0
The reaction reaches equilibrium at 1 000 °C in a closed container.
- State Le Châtelier’s principle. (2)
- How will an increase in pressure at 1 000 °C (by decreasing the volume) affect the yield of hydrogen gas? Write down only INCREASES, DECREASES OR NO EFFECT. Explain the answer. (2)
- Give TWO reasons why high temperatures are used for this reaction. (2) [6]
hint
There are various ways to state Le Châtelier’s Principle correctly.
Solution
- When the equilibrium in a closed system is disturbed the system will shift the equilibrium position (OR re-instate a new equilibrium)✓ as to favour the reaction that will oppose OR cancel OR counteract the change OR disturbance. ✓
OR
When a stress / change is placed on a system in equilibrium the system shifts the equilibrium ( position) OR re-instate a new equilibrium ✓ so as to remove OR cancel OR oppose the stress / change. ✓ (2)
OR
When the conditions affecting an equilibrium are changed, the equilibrium (position) shifts in such a way ✓ as to oppose the change OR cancel the change. ✓ (any two) - Decreases ✓ When the pressure is increased, the reverse reaction is favoured.✓
OR
The reaction that produced the smaller volume/amount of gas is favoured. ✓
OR
4 mol or volumes of gas produces ✓ 2 mol or volumes of gas. ✓ (2) (any two) - Products form at faster rate. ✓
Higher yield of products. ✓ (2) [6]
hint
In physical chemistry, saturation is the point at which a solution of a substance can dissolve no more of that substance. Additional amounts of the solute will appear as a separate phase (usually as a precipitate solid). This point of maximum concentration, the saturation point, depends on the temperature and pressure of the solution as well as the chemical nature of the substances involved.
Activity 5
1. The following hypothetical reaction reaches equilibrium in a closed container at a certain temperature:
X2(g) + Y2(g) ⇋ 2XY(g) ∆H < 0
Which ONE of the following changes will increase the AMOUNT of XY(g)?
- Decrease in temperature
- Increase in temperature
- Increase in pressure
- D. Decrease in pressure (2)
2. The equation below represents a chemical reaction at equilibrium in a closed container.
H2(g) + I2(g) ⇌ 2HI(g) ∆H < 0
Which ONE of the following changes will increase the yield of HI(g) in the above reaction?
- Increase the temperature
- Decrease the temperature
- Increase the pressure by decreasing the volume
- Decrease the pressure by increasing the volume (2)
3. A chemical reaction reaches equilibrium. Which ONE of the following statements regarding this equilibrium is TRUE?
- The concentrations of the individual reactants and products are constant.
- The concentrations of the individual reactants and products are equal.
- The concentrations of the individual reactants are zero.
- The concentrations of the individual products increase until the reaction stops. (2)
4. The reaction represented by the equation below reaches equilibrium.
2CrO24−(aq) + 2H+(aq) ⇌ Cr2O27−(aq) + H2O(ℓ)
yellow orange
Which ONE of the following changes to the reaction mixture will change its colour from yellow to orange?
- Add a catalyst.
- Add water to the reaction mixture.
- Add a few drops of sodium hydroxide solution to the reaction mixture.
- Add a few drops of concentrated hydrochloric acid to the reaction mixture. (2)
5. Give one word for the following phrase:
The stage reached in a reversible chemical reaction when the rate of the forward reaction is equal to the rate of the reverse reaction. (2) [10]
Solution
1. A ✓✓ (2)
2. B✓✓ (2)
3. A ✓✓(2)
4. D ✓✓ (2)
5. (Dynamic/Chemical) equilibrium ✓✓ (2) [10]
NB
Solids and pure liquids are omitted from the Kc expression as their concentration is [1], as multiplying by 1 has no effect.
Activity 6
Write Kc expressions for each of the following reactions:
- N2(g) + 3H2(g) ⇋ 2NH3(g) (1)
- CaCO3(s) ⇋ CaO(s) + CO2(g) (1)
- P4(s) + 6Cℓ2(g) ⇋ 4PCℓ3(ℓ) (1) [3]
Solution
- Kc = [NH3]3
[N2]2.[H2]3 - Kc = [CO2]
- Kc = 1
[CL2]6
Activity 7
The thermal decomposition of calcium carbonate (CaCO 3) is an example of a heterogeneous equilibrium. The decomposition that takes place in a closed container can be represented by the following equation:
CaCO3(s) ⇌ CaO(s) + CO2(g)
Initially 5 g of CaCO3(s) is placed in a closed 500 cm3 container and then heated. Equilibrium is reached at 900°C.
- Why is the above decomposition referred to as a heterogeneous equilibrium? (1)
- Calculate the mass of unreacted CaCO3(s) that remains in the container at equilibrium if Kc for the reaction is 0,0108 at 900°C. (19)
- It is found that the value of Kc increases when the container is heated. Is the forward reaction exothermic or endothermic? Use Le Châtelier’s principle to explain your answer. (5)
- The volume of the container is now decreased to 250 cm3 while the temperature is kept constant. How will each of the following be affected? Give a reason for your answer.
4.1 The value of Kc.
4.2 The number of mol CaCO3(s) present in the equilibrium mixture. (10)
4.3 The concentration of CO2(g) at the new equilibrium. - More CaCO3(s) is now added to the equilibrium mixture in the 500 cm3 container. How will this change influence the number of moles of CO2(g) in the equilibrium mixture? Give a reason for your answer. (2) [37]
- hint; Refer back to the definition of a heterogeneous equilibrium
Solutions
- Reactants and products are in different phases. 3 (1)
- This answer takes six steps. Answers for questions 3.3 to 3.5 appear below these steps.
Step 1:
Convert the mass of the reactant to moles. ONLY moles may be used in the table.
- M(CaCO3) = 40 + 12 + 3(16) = 100 g·mol–1
n = m = 5 = 0,05 mol
M 100
Step 2:
Write the expression for Kc and substitute all the known values – remember that the value of Kc was given.
- Kc = [CO2]
0,0108 = [CO2]
∴ [CO2] = 0,0108 mol·dm–3
Step 3:
Use the calculated CO2(g) concentration and the formula for concentration (c = n/V ) to calculate the number of moles of CO2 in the equilibrium mixture. The volume of the container (500 cm3) was given. Convert the volume to dm3. Show all working.
- c = n ∴ 0,0108 = n ∴ n = (0,5)(0,0108) = 0,0054 mol CO2
V 0,5
Step 4:
Find the column with 2 values. Complete the mol used or produced line for this compound. (0 mol CO2 initially + produced mol CO2 = 0,0054 mol
∴ mol CO2 produced = 0,0054 mol)
Step 5:
From the balanced equation ratio 1 : 1 : 1 determine the ratio of the moles used and produced. Therefore 1 : 1 : 1 becomes 0,0054 (from Step 3): 0,0054 : 0,0054
Step 6
For the reactant CaCO3 ... initial mol – mol used = final mol at equilibrium
∴0,05 mol – 0,0054 = final mol at equilibrium = 0,0046 mol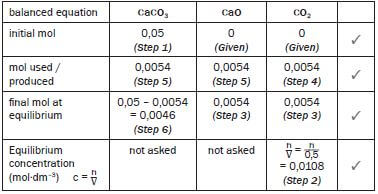
∴0,0046 mol CaCO3 remains unreacted at equilibrium
M(CaCO3) = 40 + 12 + 3(16) = 100 g·mol–1
- n = m ∴ 0,0046 = m ∴ m = (0,0046)(10) = 0,46 g
M 100
∴ 0,46g CaCO3 remains unreacted. (19)
Hint : Mass was asked, so convert the mol calculated to mass n = m
M
3. Endothermic. ✓
- Kc increases ∴ the forward reaction or reaction to the right is favoured. ✓✓
- According to Le Châtelier’s principle, an increase in temperature ✓ the forward reaction or will favour the endothermic reaction reaction to the right must be endothermic. ✓ (5)
4.1 Remains the same. Only temperature affects the value of Kc.
4.2 Increases. ✓✓
- Decreasing the volume increases the pressure. According to Le Châtelier’s Principle, when the pressure is increased, the reaction which decreases the pressure by producing fewer moles of gas products, is favoured. ✓
As 0 mol (g) ∴ 1 mol (g), the reverse reaction is favoured. ✓ - 4.3 Remains the same. ✓✓
Kc = [CO2]. The temperature remains constant ∴ Kc remains constant ∴ the concentration of CO2 will remain constant. ✓ (10)
5. Remains the same. Adding a solid does not affect the equilibrium, it does not change the concentration of a reagent. ✓✓ (2) [37]
Activity 8
NB:
- When∆H > 0 it means the reaction to the right (the forward reaction) is endothermic
- When∆H < 0 the forward reaction is exothermic
hint
- These are the initial concentrations – before the new equilibrium is established. V = 1 dm3 (given)
∴the values of the initial concentrations are equal to the initial number of moles.
c= n and c = n
V 1
∴ n = c(1)
The equation below represents an equilibrium reaction in a sealed 1dm3 container.
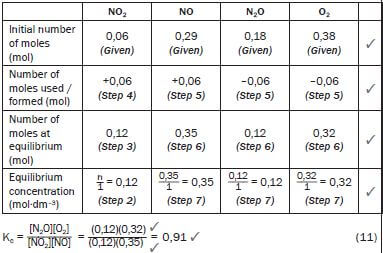
NO2(g) + NO(g) ⇋ N2O(g) + O2(g) ΔH > 0
Equilibrium was reached at a certain temperature and the value of Kc was 3,93. The concentration (in mol·dm–3) of each reactant and product in the container at equilibrium was:
- [NO2] = 0,06 mol·dm–3
- [NO] = 0,29 mol·dm–3
- [N2O] = 0,18 mol·dm–3
- [O2] = 0,38 mol·dm–3
One of the conditions affecting the equilibrium is changed and a new equilibrium is established. At the new equilibrium, the concentration of the NO2(g) is 0,12mol·dm–3.
1. Calculate the Kc value at the new equilibrium. (11)
2. Which condition, concentration or temperature, was changed? (1)
3. Give an explanation for the answer to Question 4.2. (1) [13]
Solutions
- NO2(g) + NO(g) + Energy ⇌ N2O(g) + O2(g)
∆ [NO2] = [NO2]final − [NO2]initial = (0,12 – 0,06) = 0,06 mol NO2 is formed. but NO2 is the product of the reverse reaction
∴ the reverse reaction was favoured.From the balanced reaction equation, 1 NO2 : 1 NO : 1 N2O : 1 O2
∴0,06 mol N2O + 0,06 mol O2 → 0,06 mol NO2 + 0,06 mol NO
So at equilibrium:
Temperature.
Kc was initially 3,93 (given) now it is 0,91. If the Kc value changed, the temperature must have changed – only a change in temperature can change the value of Kc. (1)
Activity 9
Consider the following reaction equation for the production of ammonia in the Haber process.
NB
- The Haber Process is the name of the chemical process to manufacture NH3
N2(g) + 3H2(g) ⇌ 2NH3(g) ∆H < 0
In each of the following cases:
- State how Kc changes and
- Briefly explain your answer.
1. The concentration of the nitrogen gas is increased. (1)
2. The pressure in the reaction system is decreased. (1)
3. FeO is added to the reaction system. (1)
4. The reaction system is heated. (5) [8]
Solutions
1. Remains constant because... ✓ (1)
2. ... Kc is only affected by a change in temperature. ✓ (1)
3. Kc Decreases ✓ (1)
4. Heating the reaction system favours the endothermic, reverse reaction ✓ which decreases the temperature ✓ of the system ∴ the concentration of the forward product, [NH3] ✓ decreases while the concentrations of the forward reactants, [N2] and [H2] increase ✓ ∴ Kc decreases. ✓ (5) [8]
Activity 10
The reaction represented by the equation below reaches equilibrium.
Co(H2O)62+(aq) + 4Cℓ–(aq) ⇋ CoCℓ42–(aq) + 6H2O(ℓ) ΔH>0
pink blue
Which ONE of the following changes to the reaction mixture will changes its colour from blue to pink?
- Add a catalyst
- Place the reaction mixture in a container with hot water.
- Add a few drops of concentrated hydrochloric acid to the reaction mixture.
- D. Add water to the reaction mixture. (2) [2]
Solution
1. D [2]
Activity 11
0,25 mol of A and 0,15 mol of B are introduced into a 1 dm3 vessel. A and B react and reach an equilibrium which can be represented by the following equation: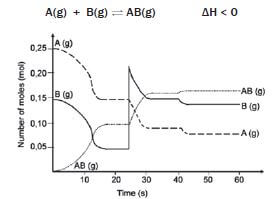
hint: Make use of the graph to read off the values
The graph shows the number of moles of A(g), B(g) and AB(g) vs time, under varying conditions.
- What are the concentrations of A(g), B(g) and AB(g) at t = 20 s? (3)
- How can we be sure that the system has reached equilibrium at t = 20 s? (1)
- 3. Give an explanation for the change that occurs at t = 25 s. (1)
- Explain the change in concentrations of A(g) and B(g) between t = 25 s and t = 35 s. (3)
- How does the equilibrium constant at t = 35 s compare with the equilibrium constant at t = 25 s? (No calculation is required.) (1)
- Assuming that the volume was kept constant, what was done at t = 40 s that produced the change as shown? Explain your answer. (5)
- Do any reactions occur during the interval t = 30 s and t = 40 s? Briefly explain your answer. (4) [18]
Solutions
- [A] = 0,15 mol·dm–3 [B] = 0,05 mol·dm–3 [AB] = 0,1 mol·dm–3 (3)
- The number of mol of A, B and AB remains constant from 16 s to 25 s. ✓(1)
- The amount of B is increased by adding B to the system, thus increasing the [B]. ✓(1)
- With the addition of B, [B] increases and the forward reaction is favoured ✓ according to Le Châtelier’s principle, so that some of B reacts with A to form more AB. Hence the [A] and [B] 3 decrease and [AB] increases. ✓ (3)
- Kc remains constant provided the temperature remains constant. ✓ (1)
- The temperature was lowered. ✓ It was not a concentration change, because there are no peaks at 25 s ✓. Therefore it must be a temperature change. From the graph, it can be seen that the forward reaction was favoured ✓ because there is an increase in the number of moles of AB, and a decrease in the number of moles of A and B when establishing the new equilibrium. ✓ According to the information given, the forward reaction is exothermic. ✓ According to Le Châtelier’s Principle, a decrease in temperature favours the exothermic reaction. (5)
- Yes, but since equilibrium has been reached, ✓ the forward and reverse reactions take place at the same time ✓ and at the same ✓ rate, so that no changes occur in the concentrations of A, B or AB. ✓ (4) [18]
Activity 12
Nitrogen and oxygen gases react in a sealed container according to the following equation:
O2(g) + N2(g) ⇋ 2NO(g)
After the reaction reaches equilibrium, certain changes are made. The following graph of rate of reaction versus time represents the situation.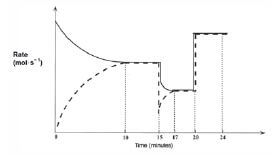
- Write the equation for the reaction represented by the dashed line on this graph. (2)
- What is represented by the section of the graph between the 10th and 15th minute? Explain your answer. (2)
- A temperature change takes place at t = 15 minutes.
3.1 Was the temperature increased or decreased at t = 15 minutes? (1)
3.2 Explain whether the forward reaction is exothermic or endothermic. (3)
3.3 What effect does this temperature change have on the equilibrium constant (Kc)? Explain your answer. (3) - A pressure change is introduced at t = 20 minutes.
4.1 Was the pressure increased or decreased? (3)
4.2 Explain how this change in pressure affects the amounts of each gas at equilibrium. (2) [16]
Solutions
- 2NO(g) → N2(g) + O2(g) ✓✓ (2)
- The system is in equilibrium ✓ because the rates of the forward and reverse reactions are equal. ✓ (2)
- 3.1 Decreased. ✓ (1)
3.2 The rates of both the forward and the reverse reactions decrease ✓, but the rate of the reverse reaction decreases more. ✓ The rate of the endothermic reaction will always decrease the most when a reaction mixture is cooled. Thus the reverse reaction is endothermic. That means that the forward reaction must be exothermic and was favoured by the decrease in temperature. ✓
O2(g) + N2(g) ⇌ 2NO(g) + Energy (3)
3.3 Increases. ✓
Since Kc = [NO]2
[N2][O2] (3)
and the forward reaction is favoured ✓ which causes an increase in [NO], the value of Kc increases. ✓ (3) - 4.1 Increased. ✓ The pressure is probably caused by a volume decrease. This results in the concentrations (c = n/V ) of the reactants, as well as the products, to increase ✓ , which in turn causes an equal increase in the forward and reverse reaction rates. ✓ (3)
4.2 The amount (n) of reactants and products does not change, ✓ since 2 moles of gaseous reactant molecules ⇌ 2 moles of gaseous product molecules. ✓ (2) [16]
Activity 13
A fertiliser company produces ammonia on a large scale at a temperature of 450°C. The balanced equation below represents the reaction that takes place in a sealed container.
N2(g) + 3H2(g) ⇌ 2NH3(g) ∆H < 0
To meet an increased demand for fertiliser, the management of the company instructs their engineer to make the necessary adjustments to increase the yield of ammonia. In a trial run on a small scale in the laboratory, the engineer makes adjustments to the TEMPERATURE, PRESSURE and CONCENTRATION of the equilibrium mixture. The graphs below represent the results obtained.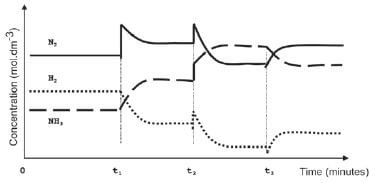
- Identify the changes made to the equilibrium mixture at each of the following times:
1.1 t1 (2)
1.2 t2 (2)
1.3 t3 (2) - At which of the above time(s) did the change made to the reaction mixture lead to a higher yield of ammonia? Write down only t1 and/or t2 and/or t3 (2)
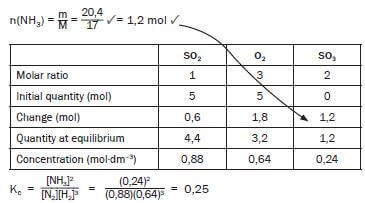
- The engineer now injects 5 mol N2 and 5 mol H2 into a 5 dm3 sealed empty container. Equilibrium is reached at 450 °C. Upon analysis of the equilibrium mixture, he finds that the mass of NH3 is 20,4 g. Calculate the value of the equilibrium constant (Kc) at 450 °C (9) [17]
Solutions
- 1.1 The concentration of nitrogen ✓ is increased ✓ / More Nitrogen ✓ added ✓ (any two) (2)
1.2 The pressure ✓ on the system is increased ✓ (2)
1.3 The temperature ✓ is increased ✓ (2) - t1 3and t2 ✓ (2)
- Pay special attention:
This is how marks are allocated to the answer of this Kc calculation:- Use of n = m/M ✓
- n(NH3) at equilibrium = 1,2 mol ✓
- Using ratio n(N2) : n(H2) : n(NH3) = 1 : 3 : 2 ✓
- n(N2) at equilibrium (initial - change) ✓
- n(H2) at equilibrium (initial - change) ✓
- divide by volume (calculation of concentration) ✓
- Kc expression ✓
- Substitution into Kc expression ✓
- Final answer ✓ (9)
 [17]
[17]
Activity 14
2 mol of NO2(g) and an unknown amount of N2O4(g) are sealed in a 2 dm3 container, that is fitted with a plunger, at a certain temperature. The following reaction takes place:
2NO2(g) ⇌ N2O4(g)
At equilibrium it is found that the NO2 concentration is 0,4 mol·dm–3. The equilibrium constant at this temperature is 2.
- Calculate the initial amount (in mol) of N2O4(g) that was sealed in the container. (8)
The plunger is now pushed into the container causing the pressure of the enclosed gas to increase by decreasing the volume - How will this change influence the amount of nitrogen dioxide at equilibrium? Only write down INCREASES, DECREASES or REMAINS THE SAME. (1)
- Use Le Châtelier’s principle to explain your answer to QUESTION 2.1.2. (2) [11]
Solutions
1.
| 2NO2 | N2O4 | |
| Initial number of mole (mol) | 2 | x |
| Number of moles used/formed (mol) | -1,2 | +0,6 |
| Number of moles at equilibrium(mol) | 0,8 | x + 0,6 |
| Equilibrium concentration (mol·dm -3) | 0,4 | (x + 0,6) 2 |
- Kc = [N2O4]
[NO2]2
2 = (x + 0,6)2
(0,4)2
x = 0,004 mol
2. Decreases (1)
3. When the pressure is increased the system will try to decrease the pressure. The forward reaction (2 mol to 1 mol) is favoured. (2) [11]
Activity 15
Name the industrial process for the production of ammonia (1) [1]
Solution
- Haber (Bosch) process [1]
Activity 16
Sulphuric acid is an important substance used in the manufacture of fertilisers. The equation below represents one of the steps in the industrial preparation of sulphuric acid.
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H < 0
- Write down the name of the process used to prepare sulphuric acid in industry. (1)
- Write down the NAME or FORMULA of the catalyst used in the process in question (1)
- Is the forward reaction exothermic or endothermic? Give a reason for the answer. The reaction reaches equilibrium at a certain temperature in a 2 dm3 closed container. On analysis of the equilibrium mixture, it is found that 0,6 mol of SO2(g), 0,5 mol of O2(g) and 0,4 mol of SO3(g) are present in the container. (1)
- List THREE changes that can be made to this equilibrium to increase the yield of SO3(g) (3)
- The temperature is NOW increased and the reaction is allowed to reach equilibrium for the second time at the new temperature. On analysis of this new equilibrium mixture, it is found that 0,2 mol of for this reaction at the new temperature. (8) [14]
Solutions
- Contact process ✓ (1)
- V2O5 (vanadium pentoxide) ✓ (1)
- Exothermic∆H < 0 ✓ (1)
- Any three: ✓✓ ✓
Decrease temperature
Increase pressure
Increase concentration of both/any one of the reactants
Remove SO3 continuously (3) -
Kc = [SO3]2 = (0,1)2 = 0,21SO2 O2 SO3 Molar ratio 2 1 2 Initial quantity (mol) 0,6 0,5 0,4 Change (mol) 0,2 0,1 0,2 Quantity at equilibrium 0,8 0,6 0,2 Concentration (mol·dm–3) 0,4 0,3 0,1
[SO2]2[O2] (0,4)2(0,3)
NB
This how marks are allocated to the answer of this Kc calculation:
- Change in n(SO3) = 0,2 mol ✓
- Ratio n(SO2) : n(O2) : n(SO3) = 2 : 1 : 2 ✓
- n(SO2) at equilibrium = initial + change ✓
- n(O2) at equilibrium = initial + change ✓
- divide three equilibrium amounts by 2 (calculation of concentration) ✓
- Kc expression ✓
- Substitution into Kc expression ✓
- Final answer ✓ (8) [14]
Activity 17
The reaction below represents the catalysed step in the contact process:
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H <0
The reaction takes place in a closed container and reaches equilibrium at 427°C. How will a HIGHER temperature affect each of the following? Write down only INCREASES, DECREASES or REMAINS THE SAME.
- The rate of production of SO3(g) (2)
- The yield of SO3(g) (2)
- The reaction is investigated on a small scale in the laboratory. Initially 4 mol of SO2(g) and an unknown mass, x, of O2(g) are sealed in a 2 dm–3 flask and allowed to reach equilibrium at a certain temperature. At equilibrium it is found that the concentration of SO3(g) present in the flask is 1,5 mol·dm–3. Calculate the mass of O2(g) initially present in the flask if the equilibrium constant (Kc) at this temperature is 4,5. (8) [12]
Solutions
1. Increases ✓✓ (2)
2. Decreases ✓✓ (2)
3.
| SO2 | O2 | SO3 | |
| Molar ratio | 2 | 1 | 2 |
| Initial quantity (mol) | 4 | x 32 | 0 |
| Change (mol) | 3 | 1,5 | 3 ratio |
| Quantity at equilibrium | 1 | x - 1,5 32 | 3 |
| Concentration (mol ·dm –3 | c = n = 1 = 0,5 V 2 | (x - 48) 64 | 3/2 = 1,5 (divide by 2) |
 (8) [12]
(8) [12]
ACIDS AND BASES QUESTIONS AND ANSWERS GRADE 12
Activity 1
1. An Arrhenius acid is a substance that
- Accept a proton
- Donates a proton
- Produces H+ in an aqueous solution
- Produces OH– in an aqueous solution (2)
2. Which of the following is an example of the strong base
- CaCO3
- KOH
- K2CO
- NaHCO3 (2)
An aqueous solution that contains more hydronium ions than hydroxyl ions is a(an)
- Acidic solution
- Neutral solution
- Basic solution
- Standardised solution (2)
A solution that has a large amount of dissolved substances in proportion to the volume of water
- Strong solution
- Weak solution
- Concentrated solution
- Diluted solution (2) [8]
Solutions
- D✓✓
- B ✓✓
- A✓✓
- C✓✓ [8]
Activity 2
1. Which of the following is the property of an acid
- Decreases H3O+ ion concentration in solution
- Decreases OH– ion concentration in solution
- Increases OH– ion concentration in solution
- Increases the pH of a solution (2) [2]
Solution
1. B✓✓ [2]
Activity 3
1. In the reaction: H2SO4(aq) + H2O(ℓ) ⇌ HSO4–(aq) + H3O+(aq), the Brønsted-Lowry bases are:
- H2O and H3O+
- H2SO4 and H3O+
- HSO4– and H3O+
- H2O and HSO4– (2) [2]
Solution
1. D ✓✓ [2]
Activity 4
Find the conjugate bases and conjugate acids.
| Acid | Conjugate base | Base | Conjugate acid |
HCℓ | Cℓ− | ||
HNO3 | NO3− | ||
H2SO4 | HSO4− | ||
HSO4− | SO42− | ||
H3PO4 | H2PO4− | ||
H2PO4− | HPO42− | ||
HPO42− | PO43− | ||
H2CO3 | HCO3− | ||
HCO3− | CO32− | ||
CH3COOH | SO42− | ||
(COOH)2 | HSO4− | ||
H2O | OH− | ||
NH4+ | NH3 | ||
H3O+ | H2O |
[28]
Note that some of these are marked in BOLD. That is because they are “amphiprotic”. We will see what this means later on.
Solution
1. Check your answers
Acid | Conjugate base |
HCℓ | Cℓ− |
HNO3 | NO3− |
H2SO4 | HSO4− |
HSO4− | SO42− |
H3PO4 | H2PO4− |
H2PO4− | HPO42– |
HPO42− | PO43− |
H2CO3 | HCO3− |
HCO3− | CO32− |
CH3COOH | CH3COO− |
(COOH)2 | C2O4H− |
H2O | OH− |
NH4+ | NH3 |
H3O+ | H2O |
Base | Conjugate acid |
Cℓ − | HCℓ |
NO3− | HNO3 |
HSO4− | H2SO4 |
SO42− | HSO4− |
H2PO4− | H3PO4 |
HPO42− | H2PO4− |
PO43− | HPO42− |
HCO3− | H2CO3 |
CO32− | HCO3− |
SO42− | HSO− |
HSO4− | H2SO4 |
OH− | H2O |
NH3 | NH4+ |
H2O | H3O+ |
[28]
Activity 5
In the acid-base equilibrium formed by adding HSO4– and OH– the acids are:
- HSO4– and H2SO4
- HSO4– and H2O
- SO42– and H2SO–
- SO42– and H2O (2)
Which of the following is amphiprotic in water?
- SO2
- SO32–
- HSO3–
- H2SO3 (2) [4]
Solutions
1. B✓✓ 2. C✓✓ [4]
Activity 6
Do you think a strong acid will have larger or smaller Ka value? Explain your answer. (3) [3]
Solution
- The strong acid will have a larger Ka value.
- A strong acid is a better proton donor, resulting in more products.
- Since the concentration of the products is in the numerator of the Ka expression, the stronger the acid, the larger the Ka. [3]
Activity 7
Choose the strongest base in the list below by comparing their Kb values.
Base Kb
- Ammonia, NH3 1,8 × 10–5
- Hydroxylamine, HONH2 9,1 × 10–9
- Ethylamine, C2H5NH2 4,3 × 10–4 (2) [2]
Solution
- C [The larger the Kb, the stronger the base] [2]
Solids and pure liquids are NEVER written in the equations for the equilibrium constant Kc, or Ka or Kb as their concentration is [1]
Activity 8
- A standard solution is a solution ...
- at 25˚C
- of an acid or a base
- of which the volume is known
- of which the concentration is known (2)
- Consider the following ionisation equilibrium:
H2O(ℓ) ⇋ H+(aq) + OH−(aq)
The ionisation constant of water (Kw) in the above reaction increases from 1 × 10–14 at 25˚C to 9,6 × 10–14 at 60˚C. Which one of the following statements is therefore correct- [H+] > [OH−] at 60˚C
- The ionisation reaction is exothermic
- The pH increases with an increase in temperature
- The pH decreases with an increase in temperature (2)
- Consider the reaction:
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
The ionisation constant for this reaction at 25°C is 1,8 × 10–5. The equilibrium constant for this reaction at 60°C, is 3,6 × 10–7. From this information we can deduce that forward reaction ...
(Explain your choice).- is endothermic
- is exothermic
- is a redox reaction
- is a precipitation reaction (2)
- Two beakers, A and B, contain solutions of the same concentration with a pH of 2 and 4,5 respectively. Which of the following combinations is correct?
Beaker A Beaker B- Weak Acid Strong Acid
- Strong Acid Weak Acid
- Strong base Weak base
- Weak base Strong base (2)
- Andile rinses the apparatus before starting an acid-base titration experiment. Which rinsing method is likely to cause inaccurate results?
- The Erlenmeyer (conical) flask is rinsed with distilled water
- The burette is rinsed with the acids it is to be filled with
- The pipette is rinsed with the base it is to be used for
- The volumetric (measuring) flask, which is used to make up the standard solution of the base, is rinsed with distilled water. (2)
- If base X is titrated against acid Y, the pH of the solution at the end point is 8. The base X and acid Y are respectively:
X Y- NaOH CH3COOH
- Na2CO3 HCℓ
- NaOH H2SO4
- Na2CO3 CH3COOH (2) [12]
Solutions
- D ✓✓(2)
- D
 (2)
(2) - B✓✓ Kc at 25°C is greater than Kc at 60°C
Kc = [CH3COO–][ H3O+]. If the mixture is heated, the Kc decreases (2) - B✓✓ Beaker A has a low pH which indicates that it contains more H+, therefore it has ionised completely. Whilst beaker B has a high pH which indicates few H+ ions, thus it has partially ionised. (2)
- A✓✓ The Erlenmeyer (conical) flask must be rinsed with distilled water. Only the number of moles of base which was measured in the pipette must be neutralised. (2)
- A✓✓ (2) [12]
Activity 9
- Write down:
1.1 The meaning of the term diprotic acid. (2)
1.2 The formula of a diprotic acid. (1) - Magnesium hydroxide (Mg(OH)2) is often used as medicine to relieve an upset stomach. The pH of the HCℓ(aq) in a person’s stomach is 1.
2.1 Calculate the concentration of the hydrochloric acid in the person’s stomach. (3)
2.2 Will the pH in the stomach INCREASE, DECREASE or STAY THE SAME after taking in a dose of Mg(OH)2? (2)
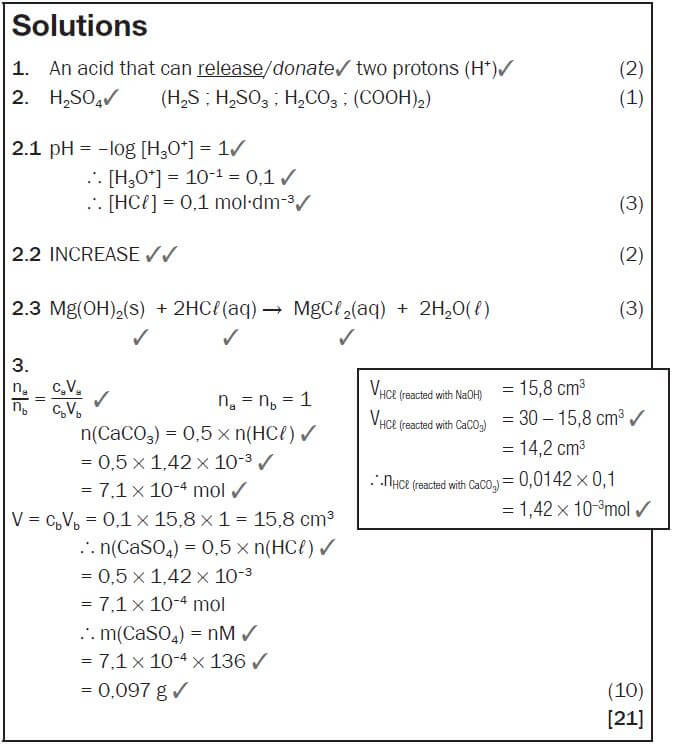
2.3 A person takes in a dose of Mg(OH)2. Write down the balanced equation for the reaction that takes place in the stomach. (3) - A textbook states that calcium sulphate (CaSO4) is slightly soluble in water. Two learners decided to test the dam water from a local municipality for calcium sulphate. They took a 0,5 dm3 sample of the dam water and treated it with sodium carbonate solution to precipitate the calcium ions present according to the following equation:
CaSO4(aq) + Na2CO3(aq) → Na2SO4(aq) + CaCO3(s)
The precipitate is then dissolved in 30 cm3 of 0,1 mol·dm–3 HCℓ solution which converts the precipitate to aqueous calcium chloride, water and carbon dioxide according to the following equation:
CaCO3 + 2HCℓ → CaCℓ2 + CO2 + H2O
The HCℓ was in excess. They neutralised the excess HCℓ by adding 15,8 cm3 of a 0,1 mol·dm–3 NaOH solution. The equation for the reaction is:
HCℓ + NaOH → NaCℓ + H2O
Calculate the mass of calcium sulphate that was present in the sample of dam water. (10) [21]
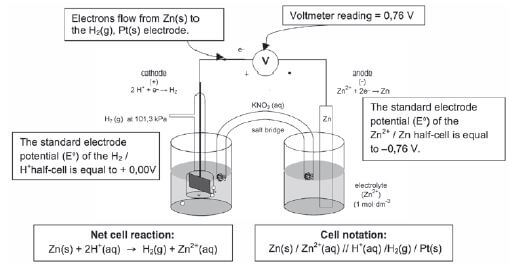
ELECTROCHEMISTRY QUESTIONS AND ANSWERS GRADE 12
Activity 1
Give one word for the following statements:
- The chemical process when an electric current is passed through an ionic compound in solution or in molten state. (1)
- An ionic solution that conducts electricity. (1)
- The reactant that donates electrons during a redox reaction. (1)
- The electrode in an electrochemical cell where reduction takes place. (1)
- TRUE OR FALSE? The reactions C(s) + O2(g) → CO2(g) and 2KCℓO3(s) → 2KCℓ(s) + 3O2(g) are examples of redox reactions. (1)
- The electrode in an electrochemical cell where oxidation occurs. (1)
- A substance that shows a decrease in oxidation number during chemical reactions. (1)
- Which of the following substances can be used as an electrolyte?
- Mercury
- Molten copper
- Sugar dissolved in distilled water
- D. Table salt dissolved in distilled water. (1) [8]
Solutions
1. Electrolysis✓ (1)
2. Electrolyte✓ (1)
3. Reducing agent✓ (1)
4. Cathode✓ (1)
5. TRUE statement✓ (1)
6. Anode✓ (1)
7. Oxidising agent✓ (1)
8. D✓ (1) [8]
Activity 2
Give ONE word for the following statements:
- The electrode in a galvanic cell at which reduction occurs. (1)
- Anode in an electrolytic cell. (1)
- The type of electrochemical cell in which electrical energy is converted to chemical energy. (1)
- TRUE or FALSE? An electrolytic cell converts mechanical energy to electrical energy. (1)
- Which ONE of the following half-reactions occurs at the cathode during the electrolysis of an aqueous CuCℓ2 solution?
- Cℓ2 + 2e– → 2Cℓ–
- Cu+ + e– → Cu
- 2Cℓ– → Cℓ2 + 2e–
- Cu2+ + 2e– → Cu (2)
- The gain of electrons by a substance in a chemical reaction is known as …
- Oxidation
- Reduction
- Electrolysis
- D. Oxidation and reduction (2) [8]
Solutions
- Cathode✓ (1)
- Positive electrode✓ (1)
- Electrolytic✓ (1)
- This statement is FALSE. This cell converts electrical energy to chemical energy.✓ (1)
- D ✓✓ (2)
- B ✓✓ (2) [8]
Activity 3
- TRUE or FALSE? During electroplating of a steel teaspoon with silver, the teaspoon is the cathode and the electrolyte is a solution of any soluble compound [2]
Solution
- FALSE. ✓… the electrolyte is a solution of a soluble silver compound. ✓ [2]
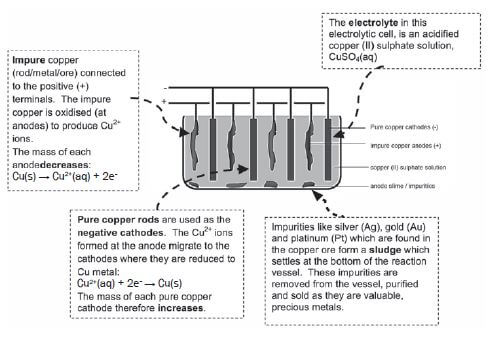
b) Refining of copper
Copper which is mined is impure and the copper ore can be refined as follows by means of electrolysis:
e.g. Worked example
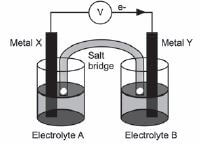
Impure copper can be purified by the process of electrolysis. The simplified diagram represents an electrolytic cell used to purify copper.
- Define the term electrolysis.
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer.
- Write down the half-reactions that take place at electrodes P and Q.
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process.
- Explain why the concentration of the copper (II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum.
- Why is the sludge of economic importance?
Solutions
- Electrolysis is a process during which electrical energy is converted to chemical energy. It is the process in which electricity is used to bring about a chemical change / decompose / break compounds into components.
- P: P is the positive electrode / anode. The impure Cu is oxidised at the positive electrode / anode.
- P: Anode: Cu(s) → Cu2+(aq) + 2e– oxidation impure
Q:Cathode: Cu2+(aq) + 2e– → Cu(s) reduction pure - Platinum and silver are both weaker reducing agents than copper and will not be oxidised to form ions.
- The rate at which copper is oxidised (at the anode) is equal to the rate at which copper ions are reduced (at the cathode).
- Silver and platinum are valuable and expensive metals and can therefore be sold at a profit.
Activity 4
- Refer to the diagram in the worked example above. One of the electrodes consists of impure copper and the other one of pure copper.
- What type of power source is used to drive the reaction in this cell? Write down only AC or DC. (1)
- Give a reason why the copper(II) sulphate is dissolved in water before it is used in this cell. (1)
When an electric current passes through the solution, electrode P becomes coated with copper. - Is electrode P the cathode or the anode? Support your answer by writing the half-reaction that takes place at electrode P. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
It is found that the impure copper plate contains platinum. The platinum forms a residue at the bottom of the container during electrolysis. - Refer to the relative strengths of reducing agents to explain why platinum forms a residue at the bottom of the container. (2)
- How will the concentration of the copper(II) sulphate solution change during electrolysis? Write down only INCREASES, DECREASES or REMAINS THE SAME. (3) [11]
Solutions
- DC ✓ (1)
- Free ions needed to conduct electricity ✓ (1)
- Cathode. ✓ Cu2+ + 2e– → Cu ✓ (2)
- Cu → Cu2+ + 2e– ✓✓(2)
- Pt is a weaker reducing agent than Cu ✓and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent than Pt and will be oxidised - Remains the same.✓ The rate at which Cu is oxidised at the anode ✓ equals the rate at which Cu2+(aq) is reduced at the cathode ✓ (3) [11]
Activity 5
Give ONE word for the following phrase:
- The main ore from which aluminium is extracted. (1)
- The name of the chemical substance in which Aℓ2O3 is dissolved to lower its melting point during the industrial extraction of aluminium. (1) [2]
Solutions
- Bauxite ✓
- Cryolite ✓ [2]
Activity 6
- In an aluminium smelter, aluminium metal is extracted from bauxite, a hydrated aluminium oxide, via an electrolytic process.
1.1 Write down the energy conversion that takes place in an electrolytic cell. (2)
1.2 Write down the equation for the half reaction responsible for the formation of aluminium metal in a smelter. (2)
1.3 Explain in terms of the relative strength of oxidizing agents why the electrolytic production of aluminum requires more electrical energy than that of iron or copper. (2)
1.4 Name TWO advantages that the use of aluminium has over that of iron. (2) - A huge aluminium smelter is planned for Coega in the Eastern Cape. When operational, it will consume 1350 MW of electricity, or 4% of the nation’s total electrical energy. It is estimated that 5200 jobs will be created at the peak of construction. About 1000 workers will be employed on a full-time permanent basis, and between 200 and 300 full-time subcontractors will also be directly associated with the smelter. (Source: www.engineeringnews.co.za; www.groundwork.org.za).
2.1 Taking the present South African socio-economic realities into account, give ONE reason why the aluminium smelter should:- Not be built (1)
- Be built (1)
2.2 Give ONE reason why environmental activists oppose the construction of the smelter. (1) [11]
Solutions
1.1 Electrical energy → chemical energy ✓✓ (2)
1.2 Aℓ3+ + 3e– → Aℓ ✓✓ (2)
1.3 Aluminium has a lower reduction potential (–1,66 V) ✓/ Weaker oxidizing agent compared to that of iron (–0,44 V) [and copper (+0,34 V)]. The aluminium ions therefore require a large amount of energy to be reduced/ will reduce more difficultly than iron (and copper). ✓ (2)
1.4 It is much lighter for the same strength (or stronger for the same mass). ✓ It is corrosion free.✓ (2)
2.1
- It will consume huge amount of electricity. ✓/ (1) Will cause power failures ✓ (any 2)
- It will create jobs ✓/Create foreign investment. ✓ (1) Contribute to GDP ✓ (any 3)
2.2 The production of the large amount of electricity used ✓ enhances the greenhouse effect (or climate change) ✓ OR The process is responsible for toxic fluoride waste OR pollution. (any one) (1) [11]
Activity 7
Give ONE word for the following phrase:
- The electrode in a galvanic cell at which reduction occurs. (1)
- The component of a galvanic cell that allows for the movement of ions between the half-cells. (1)
- Which statement is CORRECT for a Zn-Cu galvanic cell that operates under standard conditions? (Standard Conditions are defined in the tables at the beginning of this book)
- The concentration of the Zn2+ ions in the zinc half-cell gradually decreases.
- The concentration of the Cu2+ ions in the copper half-cell gradually increases.
- Negative ions migrate from the zinc half-cell to the copper half-cell.
- The intensity of the colour of the electrolyte in the copper half-cell gradually decreases. (2)
- The reactions below occur in two different electrochemical cells X and Y.
Cell X: CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Cell Y: Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Which ONE of the following correctly describes the substance that forms at the CATHODE of each of these cells?
Cell X | Cell Y | |
| A | Cℓ2(g) | Cu(s) |
| B | Cu(s) | Cu(s) |
| C | Cℓ2(g) | ZnSO4(aq) |
| D | Cu(s) | ZnSO4(aq) |
5. Which one of the following statements regarding a copper-silver galvanic cell is TRUE?
- Silver is formed at the anode
- Copper is formed at the anode
- Silver is formed at the cathode
- Copper is formed at the cathode (2) [8]
Solutions
1. Cathode ✓ (1)
2. Salt bridge ✓ (1)
3. D ✓✓ (2)
4. B ✓✓ (2)
5. C ✓✓ (2) [8]
Activity 8
- Which one of the following solutions can be stored in an aluminium container?
(Use the Table of Standard Reduction Potentials.)- CuSO4(aq)
- ZnSO4(aq)
- NaCℓ(aq)
- Pb(NO3)2(aq) (2) [2]
Solution
1. C ✓✓[2]
Activity 9
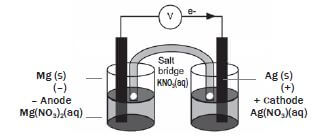
The galvanic cell represented in the diagram consists of a Mg electrode dipped into a Mg(NO3)2 solution, and a Pb electrode dipped into a Pb(NO3)2 solution. Assume that the cell operates under standard conditions.
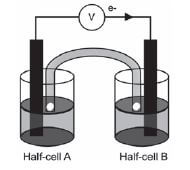
1. State TWO standard conditions under which this cell operates. (2)
2. Write down the half-reaction that takes place in half-cell A. (2)
3. Write down the cell notation for this cell. (2)
4. Calculate the emf of this cell. (3)
5. How will each of the following changes influence the value of the cell’s emf calculated in QUESTION 2.4? Write down only INCREASES, DECREASES or REMAINS THE SAME
5.1 An increase in [Mg2+(aq)] (1)
5.2 An increase in [Pb2+(aq)] (1)
6. In which direction, from half-cell A to B or from half-cell B to A, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer. (3) [14]
Solutions
1. Temperature: 298 K (25 °C) ✓✓(2)
2. Mg(s) → Mg2+(aq) +2e– ✓✓ (2)
3. Mg(s)/Mg2+(1 mol·dm–3) ✓// Pb2+(aq)(1 mol·dm–3)/Pb(s) ✓ (2)
4. E0cell = E0cathode – E0anode = - 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.
5.1 DECREASES ✓ (1)
5.2 INCREASES ✓ (1)
6.
- Half-cell A to half-cell B ✓
- Concentration of positive ions/cations ✓/ Pb2+ ions decreases in half-cell B. ✓
OR - Concentration of positive ions/cations/Mg2+ ions increases in half-cell A
- To prevent a build-up of positive ions in half-cell A and negative ions in half-cell B /for electrical neutrality.
- Positive ions migrate from/through the salt bridge (3) [14]
Activity 10
Learners conduct an investigation to determine which combination of two half-cells will provide the largest emf under standard conditions.
Three half-cells, represented as A, B and C in the table below, are available.
Half-cell A | Half-cell B | Half-cell C |
Mg/Mg2+ | Pb/Pb2+ | Aℓ/Aℓ3+ |
The learners set up galvanic cells using different combinations of the above half-cells.
- Write down the standard conditions under which these cells operate. (2)
- Write down the dependent variable in this investigation. If you can’t remember what the dependent (and independent) variables are, please see the introductory materials at the beginning of this book. (1)
- Use the Table of Standard Reduction Potentials to determine which one of the three half-cells (A, B or C) contains the:
3.1 Strongest reducing agent. (1)
3.2 strogest oxidizing agent. (1) - Without any calculation, write down the combination of two half-cells which will produce the highest emf. Write down only AB, BC or AC. (1)
- One group of learners set up a galvanic cell using half-cells A and B, as shown below. X is one of the components of the galvanic cell.

5.1 Write down the NAME or SYMBOL of the substance that will act as the anode in this cell. Give a reason for the answer. (2)
5.2 Calculate the initial emf of this cell. (3)
5.3 How will an increase in the concentration of the electrolyte in half-cell B affect the initial emf of this cell? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
5.4 Briefly explain how component X ensures electrical neutrality while the cell is functioning. (1) [13]
Solutions
- Temperature 25 °C /298 K ✓
Concentration of electrolytes = 1 mol·dm–3 ✓ (2) - emf/potential difference ✓ (1)
- 3.1 Half-cell A ✓ (1)
3.2 Half-cell B ✓ (1) - Combination AB ✓ (1)
- 5.1 Magnesium/Mg. 3Is oxidised/loses electrons/increase in oxidation number/stronger reducing agent ✓ (2)
5.2 E0cell = E0cathode – E0anode = – 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.3 Increases ✓ (1)
5.4 Allows for the migration of positive ions to the cathode half-cell ✓ (1)
OR
Allows for the migration of negative ions to the anode half-cell [13]
Activity 11
- Which is the the reference electrode in the diagram above? (1)
- TRUE or FALSE? A standard cell that has a negative emf cannot be used as a galvanic cell. (2)
- TRUE or FALSE? The standard conditions used to measure standard electrode potentials are: A temperature of 273 K, a concentration of 1 mol·dm–3, and a pressure of 101,3 kPa. (2)
- Which ONE of the following containers can be used to store an iron(II) sulphate solution?
- Aℓ
- Mg
- Ni
- Zn (2) [7]
Solutions
- Hydrogen half-cell ✓ (1)
- TRUE ✓ (2)
- FALSE. ✓ A temperature of 298 K (25 °C) — the question gives the temperature incorrectly ✓(2)
- C ✓ (2) [7]
Activity 12
Give ONE word for the following phrase:
- The process taking place in a cell when an electric current passing through its electrolyte, results in chemical reactions at its electrodes. (1)
- TRUE or FALSE? A battery labelled as 3 000 mA·h can deliver a current of 500 mA for 6 hours. (2) [3]
Solutions
1. Electrolysis ✓ (1)
2. True. ✓✓ (2) [3]
Activity 13
- The most common filling for tooth cavities is ‘dental amalgam’ – a solid solution of tin and silver in mercury. If you bite on a piece of aluminium foil that is in contact with a dental filling in your mouth, you may feel a painful sensation because
- the aluminium foil is hard.
- a temporary galvanic cell has been set up whilst the aluminium and filling are in contact.
- electrons are being transferred to the aluminium.
- a temporary electrolytic cell has been set up whilst the aluminium and filling are in contact. (2)
- Which one of the following can be classified as a redox reaction?
- NH3(g)+ HNO3(g) →NH4NO3(s)
- FeS(aq)+ 2HCℓ(aq) → FeCℓ2(aq) + H2S(g)
- Aℓ(s) + Cℓ2(g) →AℓCℓ3(s)
- Mg(NO3)2(s) → Mg2+(aq) + 2NO3–(aq) [with H2O catalyst] (2)
- A silver (Ag) spoon is left in a beaker containing copper nitrate, Cu(NO3)2, solution. What will be observed after some time?
- The spoon will be covered with a thin layer of copper.
- The nitrate will form NO2 gas and copper will form in the beaker.
- The spoon will start to erode and its mass will decrease.
- There will be no reaction.
Solutions
1. B. ✓✓ (2)
2. C. ✓✓ (2)
3. D. ✓✓ (2) [6]
Activity 14
- A group of learners set up an electrochemical cell using lead and copper half cells.
1.1 Which ONE of copper or lead will be the negative electrode? Give a reason for your answer. (2)
1.2 Use the Table of Standard Reduction Potentials and write down the reduction half-reaction that will take place in this cell. (2)
1.3 A 2V bulb is connected to the cell. Will the bulb light up? Justify your answer with a calculation. (5)
1.4 A voltmeter is now connected to the cell instead of the bulb. It is observed that after a while the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell. (3) - A cell such as the one described above is not much useful. However, the principle is used in batteries for cars, torches, computers, et cetera. These batteries are called secondary cells. One such battery is the mercury cell. The half reactions occurring in this cell are shown below.
Zn(s) + 2OH–(aq) → ZnO(s) + H2O + 2e–……… (i)
HgO(s) + H2O + 2e– → Hg(ℓ) + 2OH–(aq)….….. (ii)
2.1 Write down the overall cell reaction. (3)
2.2 Why does the use of this battery pose an environmental hazard? (1) [16]
Solutions
1.1 Lead. ✓ Stronger reducing agent ✓ OR Is oxidised preferably (2)
1.2 Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
1.3 E0cell = E0cathode − E0anode ✓ = 0,34– (–0,13) ✓ =0,47V✓ (5) Bulb will not light, ✓ energy from cell not sufficient ✓ OR emf of cell is much less than 2 V needed for the bulb ✓✓
1.4. While the cell is in operation, the concentration of the reactants (Cu2+(aq)) decreases. ✓ At the same time the concentration of the products (Pb2+(aq)) increases. ✓ The result is a gradual decrease in the cell potential until there is no further change in concentration and equilibrium is reached where the cell potential will be zero. ✓ (3)
2.1 Zn(s) + HgO(s) → ZnO(s) + Hg(ℓ) ✓✓✓ (3)
2.2 Mercury is poisonous ✓ (1) [16]
Activity 15
The discovery of electrochemical cells has revolutionised our way of life.
The diagram below represents an electrochemical cell.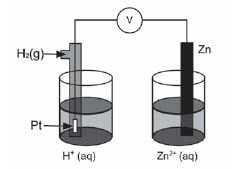
- Name the type of electrochemical cell that converts chemical energy to electrical energy. (1)
- If the electrochemical cell is set up as illustrated, there will be no reading on the voltmeter. Give a reason for this observation.(1)
- Write down the value of the standard emf of the electrochemical cell when it is functioning. (1)
- Write down the voltmeter reading when the net cell reaction in the above electrochemical cell reaches equilibrium. (1)
- Write down the equation for the reaction that occurs at the anode. (1)
- Another electrochemical cell is set up under standard conditions by replacing the standard hydrogen half-cell with a standard magnesium half-cell.
6.1 Which electrode will undergo a decrease in mass? Give a reason for your answer. (2)
6.2 Calculate the initial emf of this electrochemical cell under standard conditions. (3)
6.3 After a while the emf of this electrochemical cell decreases. Explain this observation by referring to the concentration of the electrolytes. (2) - Electrochemical cells such as motor car batteries with plastic casings can harm the environment if not disposed of safely. Suggest TWO ways how motor car batteries can be safely disposed of. (2) [14]
Solutions
1. Galvanic/voltaic cell ✓ (1)
2. Incomplete circuit/No salt bridge ✓ (1)
3. 0,76 V ✓ (1)
4. Zero ✓ (1)
5. Zn(s) → Zn2+(aq) + 2e– ✓ (1)
6.1 Mg. ✓ Mg is oxidised ✓ (2)
6.2 E0cell = E0cathode – E0anode ✓ = 0,76 – (–2,36) ✓ = 1,6 V ✓ (3)
6.3 As the cell functions, the concentration of zinc ions (reactants) decreases ✓ relative to the standard conditions and the concentration of magnesium ions (products) increases relative to standard conditions. The reverse reaction starts opposing the forward reaction causing the emf to decrease relative to standard conditions. ✓ (2)
7. Neutralise acid before disposal. ✓ Recycle plastic casing and lead electrodes ✓ (2) [14]
Activity 16
In 1780, Luigi Galvani discovered that when copper and zinc metal were connected to each other and if each free end touched different parts of the same nerve of a frog leg at the same time, the frog’s leg contracted. He called this “animal electricity”.
- Briefly explain what this “animal electricity” really was. (1)
The diagram shows an electrochemical cell setup under standard conditions using aluminium (Aℓ) and nickel (Ni) electrodes. AℓCℓ3(aq) and NiCℓ2(aq) are used as the electrolytes, and a solution of sodium nitrate (NaNO3(aq)) is used in the salt bridge.
Answer each of the following questions on this electrochemical cell:
- The diagram indicates that electrons flow from metal X to metal Y.
Identify:
2.1 Metal X (1)
2.2 Electrolyte B (1) - What is the concentration of electrolyte B? (1)
- Write down the FORMULA of the substance that moves towards metal Y in the salt bridge. (1)
- Write down the half-reaction that occurs at the cathode of this cell. (2)
- Calculate the reading on the voltmeter. (3)
- State what happens to the concentration of metal ions in the solution containing electrolyte as time goes by? (1)
- 8.1 Consider 5.7 again and recall your answer. What effect does this have on the voltmeter reading? (1)
8.2 Briefly explain your answer to 5.8.1. (2) [14]
Solutions
- The chemical reaction between the zinc and the copper r eleased electrical energy ✓ (1)
- 2.1 Metal X: Aℓ ✓ (1)
2.2 Electrolyte B: NiCℓ2 ✓ (1) - 1mol·dm–3 ✓ (1)
- NO3– ✓ (1)
- Ni2++ 2e– → Ni ✓ ✓ (2)
- E0cell = E0oxidising agent − E0reducing agent ✓ = –0,25V–(–1,66V) ✓ =1,41V✓ (3)
- Increase ✓ (1)
- 8.1 The voltmeter reading decreases ✓ (1)
8.2 As the [Aℓ3+] increases, ✓ the reverse reaction in the Aℓ Aℓ3+ half-cell:
Aℓ ⇌ Aℓ3+ +3e– is favoured.✓ (2)
OR
The tendency of the net reaction Aℓ + Ni2+ → Aℓ3+ + Ni to proceed from left to right is reduced. This lowers the electrode potential of this half-cell, resulting in a lower cell potential. [14]
Activity 17
Tina wants to investigate the effect of the area of the metal plates used as electrodes in a galvanic cell on the emf of the cell. She sets up the following Zn/Pb cell under standard conditions and measures the emf.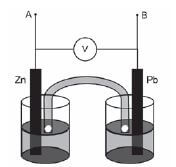
- Which electrode will show an increase in mass when this cell is functioning? (1)
- Write down the equation for the half-reaction occurring at the anode. (2)
- Calculate the emf that you would expect Tina to read on the voltmeter. (3)
- Name TWO variables that should be controlled for during this investigation. (2)
- Tina now replaces the two metal plates with ones of larger surface area, and takes the readings again.
5.1 How would you expect the new emf to compare with the one calculated in Question 6.3? (Only write SMALLER THAN, LARGER THAN or EQUAL TO) (1)
5.2 Explain your answer to Question 5.1 (1) - Tina now connects a resistor of low resistance across terminals A and B. She notes that the reading on the voltmeter immediately drops.
6.1 Give a reason for this observation. (1)
6.2 After some time she observes a further drop in the reading on the voltmeter. Give a reason for this observation. (2) [13]
Solutions
- Pb ✓
- Zn → Zn2+ + 2e– ✓✓
- E0cell = E0oxidising agent − E0reducing agent✓ –0,13 – (–0,76) ✓ =0,63V✓ (3)
- Temperature, ✓ (initial) concentration of electrolytes ✓(2)
- 5.1 Equal to ✓ (1)
5.2 Area/size of electrodes has no effect on the emf of a cell. It is still a standard cell ✓ (1) - 6.1 the cell has internal resistance ✓ (1)
6.2 The emf decreases as the concentration of Pb2+(aq) decreases. ✓/The cell is running flat as the electrolyte concentration in the Pb cell decreases. ✓✓ (any 2) (2) [13]
Activity 18
Electrolysis is an important industrial process used to decompose compounds, extract metals from their ores and to purify metals like gold or copper.
The simplified diagram below represents an electrolytic cell used to purify copper.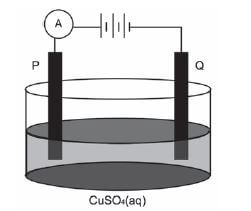
- Define the term electrolysis. (2)
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process. (2)
- Explain why the concentration of the copper(II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum. (2)
- Why is the sludge of economic importance? (1) [11]
Solutions
- The process in which electricity is used to bring about a chemical change / decompose / break compounds into components ✓✓(2)
OR
A process in which electrical energy is converted to chemical energy. - P. ✓
P is the positive electrode /anode ✓ (2)
OR
Oxidation takes place at the positive electrode/anode - Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
- Pt and Ag are both weaker reducing agents ✓ (than copper) and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent (than Pt and Ag) and will be oxidised - The rate at which copper is oxidised at the anode, ✓ is equal to the rate at which copper ions are reduced at the cathode. ✓ (2)
- Contains platinum and silver that are valuable/expensive metals ✓ (1) [11]
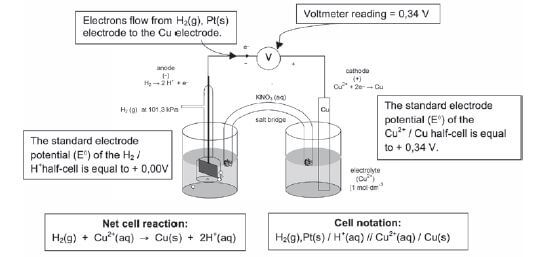
THE CHLOR-ALKALI INDUSTRY QUESTIONS AND ANSWERS GRADE 12
Activity 1
The electrolysis of saturated sodium chloride can be illustrated as follows:
Hydrogen and chlorine bubble off at the electrodes.
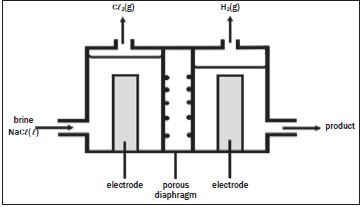
- Give an equation to show how chlorine bubbles are formed at the electrode. (2)
- At which electrode are the chlorine bubbles formed? (1)
- At which electrode does hydrogen gas form? (1)
- What is the name of the product which leaves the cell? (1)
- Give TWO applications of the product formed in 2.4. (2)
- What purpose does the porous diaphragm serve? (2)
- The chlorine gas produced, dissolves in water to form chlorine water. Write down a balanced equation for the reaction that takes place. (2) [11]
Solutions
- 2Cℓ–(aq) → Cℓ2(g) + 2e– (2)
- Anode (1)
- Cathode (1)
- Sodium hydroxide (1)
- Making soap and detergents; paper; rayon and other fibres; dyeing textiles (any 2) (2)
- Stops chlorine passing through; helps to separate sodium hydroxide from NaCℓ(aq). (2)
- Cℓ2 + H2O → HCℓ + HOCℓ (2) [11]
Activity 2
The simplified diagram of a cell used in the chlor-alkali industry is shown below.
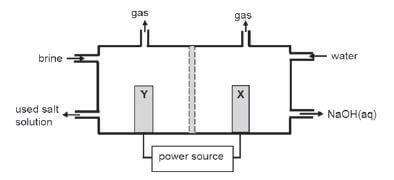
- Write down the CHEMICAL FORMULA of brine. (1)
- At which electrode, X or Y is chlorine gas formed? (1)
- Write down a half-reaction that explains the formation of hydrogen gas at one of the electrodes. (4)
- The purity of the sodium hydroxide produced in the chlor-alkali industry depends on the extent to which it is separated from the chlorine gas produced by this cell. Briefly describe how chlorine gas and sodium hydroxide are prevented from mixing in this cell. (2)
- Apart from the advantages and disadvantages of products produced, write down for this process:
5.1 ONE positive impact on humans. (1)
5.2 ONE negative impact on humans (1) [10]
Solutions
- NaCℓ (aq) (1)
- Y (1)
- 2H2O + 2e– →H2+2OH – (4)
- The membrane prevents chloride ions from moving to the cathode, only allows positive ions through. (2)
- 5.1 Job creation resulting in more people having a better life. (1)
5.2 Uses huge amounts of electricity resulting in load shedding.
OR
Chemical plant uses a lot of space that could have been used for housing/gardens, etc. (any one) (1)
THE FERTILISER INDUSTRY QUESTIONS AND ANSWERS GRADE 12
Activity 1
- Which of the following is a primary mineral nutrient that is needed by plants?
- N
- C
- Mg
- Na (2) [2]
Solution
1. A [2]
Activity 2
- Which ONE of the following is NOT associated with eutrophication in water?
- Dead zones
- Algal bloom
- Depletion of oxygen
- Increased aquatic life (2) [2]
Solution
- D [2]
Activity 3
Fertilisers allow farmers to grow crops in the same soil year after year. However, environmental problems, such as eutrophication, are associated with the application of fertilisers.
- State ONE PRECAUTION that a maize farmer can take to prevent eutrophication. (1)
Nitric acid is an important reactant in the production of ammonium nitrate, a nitrogen-based fertiliser. - Write down the name of the industrial process for the production of nitric acid. (1)
- Write down a balanced equation for the preparation of ammonium nitrate from nitric acid. (3) [5]
Solutions
- Use fertilisers sparingly / Do not over-fertilise
Make use of precision (computerised) application of fertilisers
Ensure that water from fields does not run into rivers/dams
Redirect water from fields into reservoirs/away from rivers/dams (any one) (1) - Ostwald process (1)
- HNO3 + NH3 → NH4NO3 (3) [5]
Activity 4
The rapidly increasing human population is resulting in an ever-increasing demand for food. To meet this demand, farmers apply fertiliser to the same cultivated land EACH YEAR.
- Explain why farmers have to apply fertilisers to their land each year. (1)
- Write down one negative impact that over-fertilisation can have on humans. (1)
- Sulphuric acid is an important substance used in the manufacture of fertilisers.
The equation below represents one of the steps in the industrial preparation of sulphuric acid.
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H<0
3.1 Write down the name of the process used to prepare sulphuric acid in industry (1)
3.2 Write down the name or the formula if the catalyst used in 2.3.1 (1)
3.3 Is the forward reaction endothermic or exothermic? Give a reason for your answer. (1)
3.4 Write down the name or formula of the fertiliser formed when sulphuric acid reacts with ammonia. (2) [7]
Solutions
- Fertilisers replenish nutrients depleted by the growing of crops (1)
- Damage to crops/soil resulting in small or no harvest/ less income. Excessive fertiliser seeps into groundwater and contaminates drinking water or runs into rivers and/or dams and causes eutrophication which may result in less income /famine/starvation /poor quality drinking water /fewer recreation areas/ environmental damage/ death of wild animals (any one) (1)
- 3.1 Contact process (1)
3.2 V2O5 vanadium pentoxide (any one) (1)
3.3 Exothermic as∆H < 0 (1)
3.4 (NH4)2SO4 OR / Ammonium sulphate (2) [7]
Activity 5
Ammonia, ammonium nitrate and ammonium sulphate are three important nitrogen-containing fertilisers. The flow diagram below shows how these fertilisers are produced in industry.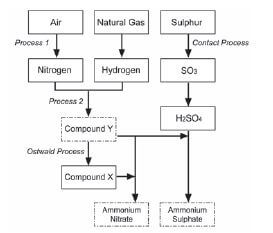
- Use the information in the flow diagram above and write down the following:
1.1 Name Process 1 (1)
1.2 Balanced equation for Process 2 (3)
1.3 Name or Formula for compound X (1)
1.4 Balanced equation for the preparation of ammonium sulphate using sulphuric acid and compound Y (3)
1.5 Name or Symbol of the primary nutrient in ammonium sulphate (1) - Write down one positive impact of fertilisers on humans (1)
- Write down two negative impacts of the use of ammonium nitrate as fertiliser, on humans. (2) [12]
Solutions
- 1.1 Fractional distillation of liquid air (1)
1.2 N2 + 3H2 → 2NH3 (reactants products balance (3)
1.3 Nitric acid / HNO3 (1)
1.4 H2SO4 + 2NH3 → (NH4)2SO4 (reactants products balance (3)
1.5 Nitrogen / N (1) - Enhance growth of crops/plants to produce more food for humans food security for humans production / application of fertiliser results in job creation selling of fertilisers stimulates the economy (any one) (1)
- (Excessive) nitrates in water (eutrophication) can result in blue baby syndrome or cancer (Excessive) nitrates/ammonium ions in water can result in poor quality drinking water or death of fish or less food or fewer recreational facilities or famine due to killing plants / crops from the excess or excessively changing the pH of the soil and thereby reducing the food production (any two) (2) [12]
THE FERTILISER INDUSTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
- Nutrients required by plants
- Functions and sources of the primary mineral nutrients
- The industrial production of fertilisers
- Flowchart: The industrial production of fertilisers
- N:P:K fertilisers
- Excessive use of fertiliser and the environment
- Alternatives to inorganic fertilisers
The fertiliser industry
The need for fertilisers is increasing due to:
- the growth in the world population – more people means more food is necessary and
- the decrease in available agricultural land – less land means the available land must produce a lot of high quality food at a high rate (fast).
Nutrients in the soil are used by the plants that grow and these nutrients must be replaced.
Inorganic fertilisers are produced industrially in the Haber, Contact & Ostwald processes at chemical plants like SASOL.
7.1 Nutrients required by plants
| MACRO NUTRIENTS | MICRONUTRIENTS | |||
Primary non- mineral nutrients | Primary mineral nutrients | Secondary nutrients | Trace elements | |
| ELEMENTS |
|
|
|
|
| SOURCES |
|
|
|
|
| AVAILABILITY |
|
|
|
|
NB: DEFINITIONS
- Promote: to help or encourage
- Essential: absolutely necessary
- Inorganic: not produced by a living organism; not containing carbon
- Mineral: an inorganic solid substance that occurs naturally (not man-made)
- Inert: unreactive
- Sufficient: enough
Did you know?
- Bone meal is produced when the bones of dead animals are ground into powder.
- Kelp meal is dried seaweed.
- Granite meal is finely ground granite rock.
Activity 1
- Which of the following is a primary mineral nutrient that is needed by plants?
- N
- C
- Mg
- Na (2) [2]
Solution
1. A [2]
7.2 Functions and sources of the primary mineral nutrients
| PRIMARY MINERAL NUTRIENTS | |||
| Nutrient | (N) | Phosphorous (P) | Potassium (K) |
| Function |
|
|
|
| Sources Pre- World War II |
|
|
|
|
|
| |
|
|
| |
Atmospheric nitrogen is fixed into compounds containing nitrates (NO3−) or ammonium ions (NH4+) so that the nitrogen can be taken in by plants and used as nutrient.
- Atmospheric fixation: fixation by lightning;
- Biological fixation: fixation by bacteria in the ground and by the roots of legumes;
- Industrial fixation: fixation by industrial processes like the Haber process.
hint
- “Fixed” means “attached”.
- In the Haber process:
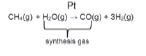
Steam reforming of methane (natural gas) in the presence of a platinum catalyst to form a mixture of carbon monoxide (CO) and hydrogen (H2) gases (synthesis gas).
- In the Contact process:
2.1 If SO2(g) is not dried before step 2, it reacts with water to form sulphurous acid, H2SO3(aq) which forms acid rain, a type of rain which is produced as a side-effect of human industrial activities, which is corrosive to structures and harmful to living things.
SO2(g) + H2O(ℓ) → H2SO3(aq)
2.2 SO3(g) reacts with water to form gaseous sulphuric acid, H2SO4(g) which escapes into the atmosphere and forms acid rain.
SO3(g) + H2O(ℓ) → H2SO4(g) - In the Contact process:
3.1 The 1st step is known as the catalytic oxidation of ammonia.
Pt
4NH3(g) + 5O2(ℓ) → 4NO(g) + 6H2O(g)
3.2 NO2 (nitrogen dioxide) is a brown gas which reacts with water to form HNO3(g) which escapes into the atmosphere and forms acid rain.
Fluorapatite, Ca5(PO4)3F
- is mined at Phalaborwa;
- is insoluble in water and can’t be absorbed by plant roots;
- reacts with sulphuric acid, H2SO4(ℓ) to produce phosphoric acid, H3PO4(aq).
Superphosphate
- is produced when fluorapatite reacts with sulphuric acid H2SO4 and is a mixture of Ca(H2PO4)2·H2O and CaSO4 (gypsum)
Triple Superphosphate
- is produced when fluorapatite reacts with phosphoric acid H3PO4 to form Ca(H2PO4)2·H2O (no CaSO4 is formed).
7.3 The industrial production of fertilisers
| Organic | |||||
| Fertiliser | Ammonium sulphate | Ammonium nitrate | Monoammonium phosphate (MAP)
| Superphosphate
| Urea (NH2)2CO |
| Reactants | NH3(g) H2SO4(ℓ) | NH3(g) Ammonia HNO3(aq) | NH3(g) Ammonia H3PO4(aq) | Ca5(PO4)3F fluorapatite H2SO4 | NH3(g) Ammonia CO2(g) |
| Reaction equation | 2NH3(g) + H2SO4(ℓ) → (NH4)2SO4(aq) | NH3(g) + HNO3(aq) → NH4NO3(aq) | NH3(g) + H3PO4(aq) → NH4H2PO4(aq) 2NH3(g) + H3PO4(aq) → (NH4)2HPO4(aq) | Not required | 2NH3(g) + CO2(g) → (NH2)2CO(g) + H2O(g) |
| Properties |
|
|
|
|
|
7.4 Flowchart: The industrial production of fertilisers
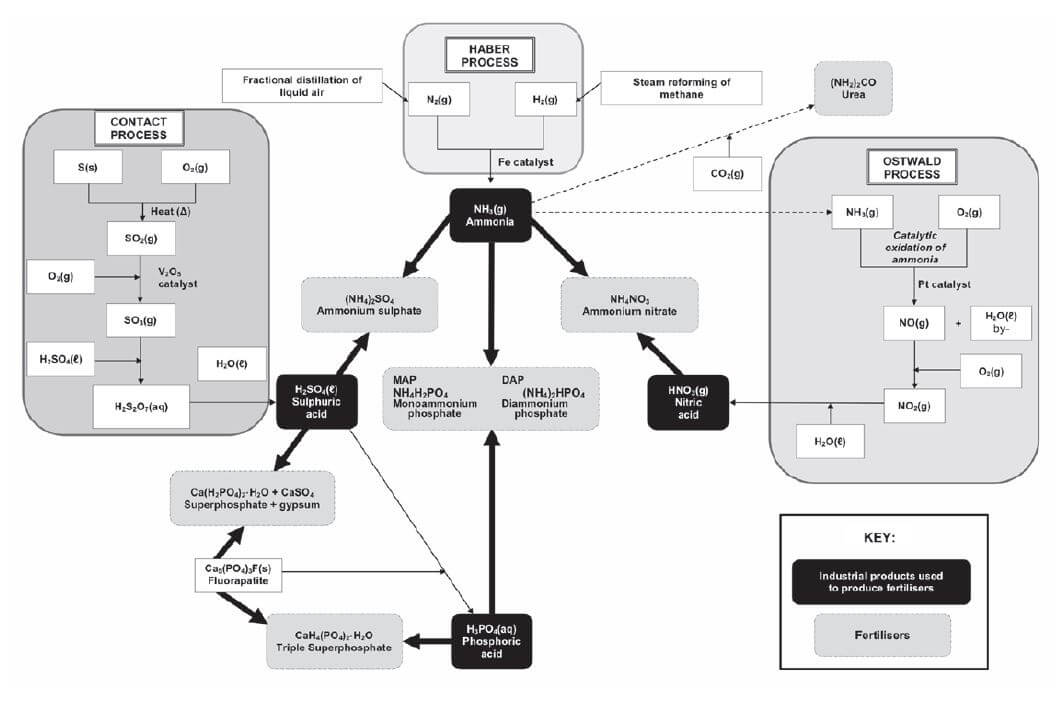
e.g. Worked example 1
Study the diagram below that illustrates the industrial preparation of nitric acid and answer the questions that follow.
- 1.1 Write down a balanced chemical equation for the formation of product A.
1.2 Name the catalyst used during the reaction in Question 2.1.
1.3 What name is given to the reaction in Question 2.1? Give a reason for this name.
1.4 Write down a balanced chemical equation for the formation of product B.
1.5 Name the products A and B.
1.6 Write down a balanced chemical equation for the formation of product C.
1.7 Name product C. - Ammonium nitrate is important for its use in fertilisers and explosives. It can be prepared by the reaction of nitric acid and ammonia.
2.1 Write down a balanced equation for the preparation of ammonium nitrate.
2.2 What kind of fertiliser is ammonium nitrate?
2.3 Which characteristic of ammonium nitrate makes it suitable for use as a fertiliser?
2.4 Which characteristic of ammonium nitrate makes it suitable to be used in explosives? - Ammonium sulphate is often used as a fertiliser to supplement nitrogen and sulphur shortages in plants.
3.1 Compile a flow chart that indicates all the industrial steps for the preparation of ammonium sulphate from air, natural gas and sulphur.
3.2 Give balanced equations for all the reactions that take place during the preparation of ammonium sulphate from air, methane and sulphur.
Solutions
- 1.1 Pt
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
1.2 Platinum or Rhodium
1.3 Catalytic oxidation of ammonia
1.4 2NO(g) + O2(g) → 2NO2(g)
1.5 A: Nitrogen monoxide B: Nitrogen dioxide
1.6 3NO2(g) + H2O(ℓ) → 2HNO3(aq) + NO(g)
1.7 Nitric Acid - 2.1 HNO3 + NH3 → NH4NO3
2.2 Primary mineral nutrient
2.3 High nitrogen content per mass
2.4 Nitrogen bonds are easily broken and therefore it decomposes rapidly and easily (explodes). - Answers for 3.1 and 3.2
- O2 + N2 (air)
- CH4 (methane)
- S
- To produce: (NH4)2SO4 (ammonium sulphate)
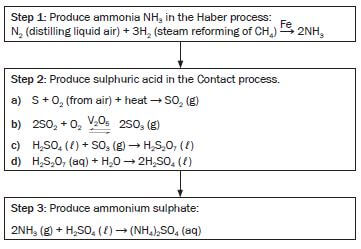
7.5 N:P:K fertilisers
As plants need a large amount of the primary mineral nutrients (nitrogen, phosphorus and potassium) and these need to be replenished (replaced) in the soil to make sure that crops grow well to provide enough food, NPK fertilisers that contain a mix of these nutrients, are usually used. They are produced by mixing a nitrogen fertiliser, a phosphorus fertiliser and potassium chloride (KCℓ).
Step by step
Step 1. Find the mass of the bag.
Step 2. Find the percentage (%) of fertiliser in the bag (the number in brackets).
- 26% fertiliser in bag
∴ 74% inert compounds
Step 3. Find the mass of fertiliser in the bag.
- mass of fertiliser = percentage fertiliser × mass of bag
100
= 26 × 10Kg = 2,6 Kg
100
Step 4. Find the total number of parts of nutrients in the bag. (Add the numbers representing the ratios of each of the elements, N, P and K.)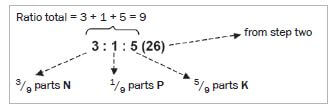
Step 5. For each element (N, P and K) find the percentage of the element in the fertiliser:
- % element = ratio element × % fertiliser
- %N= ratio N ×% fertiliser = 3 × 26 = 8,67
total ratio 9 - %P= ratio P ×% fertiliser = 1 × 26 = 2,89
total ratio 9 - %P= ratio K ×% fertiliser = 5 × 26 = 14,44
total ratio 9
Step 6. For each element (N, P and K) find the mass of that element in the bag.
- mass element = ratio element × % fertiliser × mass of bag
total ratio 100- 3 × 26 × 10 kg = 0,87 kg
9 100 - 1 × 26 × 10 kg = 0,29 kg
9 100 - 5 × 26 × 10 kg = 1,44 kg
9 100
- 3 × 26 × 10 kg = 0,87 kg
The per component totals (0,87; 0,29; 1,44) add up to 2.6kg.
e.g. Worked example 2
A bag of fertiliser has the following information on it: 3 : 2 : 3 (26).
- What information can you deduce from these numbers?
- Calculate the percentage composition of the fertiliser in the bag.
Solutions
- It shows the ratio N : P : K.
3 + 2 + 3 = 8therefore the fertiliser mixture consists of:
3/8 parts N; 2/8 parts P and 3/8 parts K
These nutrients make up 26% of the mass of the bag content. The other 74% is made up of gypsum, lime and sand. - %N= ratio N ×% fertiliser
total ratio- =3 × 26 = 9,75
8 - = 2 × 26 = 6,5
8 - = 3 × 26 = 9,75
8
- =3 × 26 = 9,75
7.6 Excessive use of fertiliser and the environment
The correct application of fertiliser to crops is essential for high quality, fast growing crops but using too much or unnecessary fertiliser has a negative effect on the environment.
- Groundwater is contaminated by fertiliser leaching (spreading by water) into the ground.
- Soil becomes acidic (pH decreases). Many plants do not grow in acidic soil.
- Invasive plants grow excessively while indigenous plants die. Invasive plants are undesirable non-indigenous (foreign) plants that grow too fast and out-compete local plants.
- Fertiliser in dams and rivers leads to “eutrophication”, defined below.
- High nitrate concentrations in drinking water decreases the ability of haemoglobin in the blood to carry oxygen and leads to ‘blue baby syndrome’.
7.6.1 Eutrophication
| In water (dams and rivers) | On land | |
| Causes |
| |
|
| |
| Effexts |
|
|
| Possible solutions |
|
|
NB: REMEMBER
An overabundance (excess, too much) of nutrients (N and P) in water leads to:
- the fast and excessive growth of algae and other water plants (this is called ‘algal bloom’) and bacteria
- causing a drop in the oxygen concentration in the water and
- the death of fish and other living organisms in the water.
hint: VOCABULARY
- Overabundance / excess: more than is needed
- Shortage: not enough
- Invasive plants: plants that do not naturally grow in a certain area
- Indigenous plants: plants that grow naturally in (are native to) a certain area
- Swamps: ground that is uncultivated but is usually covered with water
- Erosion: soil and rock is removed from the Earth’s surface by wind, rain or water
- Stunted growth: reduced growth leading to smaller, underdeveloped plants
Activity 2
- Which ONE of the following is NOT associated with eutrophication in water?
- Dead zones
- Algal bloom
- Depletion of oxygen
- Increased aquatic life (2) [2]
Solution
- D [2]
7.6.2 The effect of a shortage or excess of the primary nutrients (N, P and K) on plants
| N (nitrogen) | P (phosphorus) | K (potassium) | |
| Shortage |
|
|
|
| Excess |
|
|
|
7.7 Alternatives to inorganic fertilisers
Although fertilisers are essential for the fast growth of high quality crops, the negative effects of inorganic compounds on the environment must be taken into account. Alternative sources of organic nutrients that can be used to ensure good crops are:
- Bone meal;
- Animal manure;
- Natural plant compost;
- Bat guano (faeces);
- Fish emulsions;
- Kelp meal
Advantages of organic fertilisers:
- Break down and release nutrients more slowly than inorganic fertilisers, so there is less chance of the fertiliser leaching into the soil and causing contamination of groundwater;
- Usually cost less and
- Are often available for free.
Disadvantages of organic fertilisers:
- Not enough is available for large scale usage;
- Provide less nutrients – more has to be used;
- Slow release of nutrients sometimes harms plants;
- Slow release may cause nutrients to be available too late in the plant’s growth cycle.
Activity 3
Fertilisers allow farmers to grow crops in the same soil year after year. However, environmental problems, such as eutrophication, are associated with the application of fertilisers.
- State ONE PRECAUTION that a maize farmer can take to prevent eutrophication. (1)
Nitric acid is an important reactant in the production of ammonium nitrate, a nitrogen-based fertiliser. - Write down the name of the industrial process for the production of nitric acid. (1)
- Write down a balanced equation for the preparation of ammonium nitrate from nitric acid. (3) [5]
Solutions
- Use fertilisers sparingly / Do not over-fertilise
Make use of precision (computerised) application of fertilisers
Ensure that water from fields does not run into rivers/dams
Redirect water from fields into reservoirs/away from rivers/dams (any one) (1) - Ostwald process (1)
- HNO3 + NH3 → NH4NO3 (3) [5]
Activity 4
The rapidly increasing human population is resulting in an ever-increasing demand for food. To meet this demand, farmers apply fertiliser to the same cultivated land EACH YEAR.
- Explain why farmers have to apply fertilisers to their land each year. (1)
- Write down one negative impact that over-fertilisation can have on humans. (1)
- Sulphuric acid is an important substance used in the manufacture of fertilisers.
The equation below represents one of the steps in the industrial preparation of sulphuric acid.
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H<0
3.1 Write down the name of the process used to prepare sulphuric acid in industry (1)
3.2 Write down the name or the formula if the catalyst used in 2.3.1 (1)
3.3 Is the forward reaction endothermic or exothermic? Give a reason for your answer. (1)
3.4 Write down the name or formula of the fertiliser formed when sulphuric acid reacts with ammonia. (2) [7]
Solutions
- Fertilisers replenish nutrients depleted by the growing of crops (1)
- Damage to crops/soil resulting in small or no harvest/ less income. Excessive fertiliser seeps into groundwater and contaminates drinking water or runs into rivers and/or dams and causes eutrophication which may result in less income /famine/starvation /poor quality drinking water /fewer recreation areas/ environmental damage/ death of wild animals (any one) (1)
- 3.1 Contact process (1)
3.2 V2O5 vanadium pentoxide (any one) (1)
3.3 Exothermic as∆H < 0 (1)
3.4 (NH4)2SO4 OR / Ammonium sulphate (2) [7]
Activity 5
Ammonia, ammonium nitrate and ammonium sulphate are three important nitrogen-containing fertilisers. The flow diagram below shows how these fertilisers are produced in industry.
- Use the information in the flow diagram above and write down the following:
1.1 Name Process 1 (1)
1.2 Balanced equation for Process 2 (3)
1.3 Name or Formula for compound X (1)
1.4 Balanced equation for the preparation of ammonium sulphate using sulphuric acid and compound Y (3)
1.5 Name or Symbol of the primary nutrient in ammonium sulphate (1) - Write down one positive impact of fertilisers on humans (1)
- Write down two negative impacts of the use of ammonium nitrate as fertiliser, on humans. (2) [12]
Solutions
- 1.1 Fractional distillation of liquid air (1)
1.2 N2 + 3H2 → 2NH3 (reactants products balance (3)
1.3 Nitric acid / HNO3 (1)
1.4 H2SO4 + 2NH3 → (NH4)2SO4 (reactants products balance (3)
1.5 Nitrogen / N (1) - Enhance growth of crops/plants to produce more food for humans food security for humans production / application of fertiliser results in job creation selling of fertilisers stimulates the economy (any one) (1)
- (Excessive) nitrates in water (eutrophication) can result in blue baby syndrome or cancer (Excessive) nitrates/ammonium ions in water can result in poor quality drinking water or death of fish or less food or fewer recreational facilities or famine due to killing plants / crops from the excess or excessively changing the pH of the soil and thereby reducing the food production (any two) (2) [12]
THE CHLOR-ALKALI INDUSTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
- Summary
- Definitions and terminology
- The chlor-alkali industry – reactants and products
- The chlor-alkali industry – industrial process
The chlor-alkali industry
The chlor-alkali industry is a large worldwide multimillion industry which produces compounds that are essential to human existence. The industry is based on the electrolysis of sodium chloride solutions.
6.1 Summary
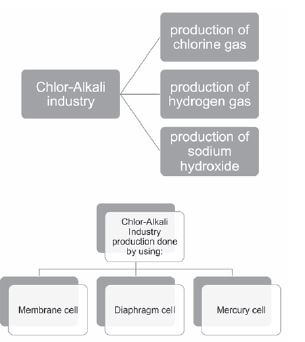
Chlor-Alkali industry involves the electrolysis of a sodium chloride solution which produces:
- chlorine gas;
- hydrogen gas and
- sodium hydroxide (aq).
6.2 Definitions and terminology
The processes involved are electrolysis, oxidation and reduction.
Electrolysis:
- is an endothermic reaction
- involves a process during which electric energy is converted to chemical energy or brings about a chemical change
- involves a non-spontaneous redox reaction.
Oxidation:
- takes place when electrons are given off
- at the anode.
Reduction:
- takes place when electrons are received
- at the cathode.
Terminology:
- Corrosive: Substance that damages and destroys substances (including living matter) it comes into contact with (touches).
- Leaching: Movement of a substance (like NaOH (aq), pesticides or fertilisers) that are carried by water, down through soil (ground) to the underground water table. The water table is the level below which the ground is saturated with water.
- Carbon footprint: The amount of carbon dioxide and other greenhouse gases released into the atmosphere due to the activities of a person, organisation or process.
- Brine: A concentrated solution of NaCℓ(aq)

You must know how to:- Identify the processes involved
- Give a description of the concept or term
- Give the correct definition
- Describe the process and chemicals involved/ produced
- State uses of the products
- State the environmental issues
6.3 The Chlor-alkali industry – reactants and products
| REACTANTS | |||
| |||
PRODUCTS | |||
| Chlorine gas Cℓ2(g) | Hydrogen gas H2(g) | Sodium hydroxide (caustic soda) NaOH(aq) | |
|
|
| |
| USES | Reactant for production of:
Used to:
| Reactant for production of:
| Reactant for production of:
Used to:
|
NB: REMEMBER:
The membrane cell is preferred because the:
- components of the cell are non-toxic;
- products are more pure and
- production costs are relatively low.
- Sodium chloride (table sale) NaCℓ(s) is an ionic compound and is very soluble in water (a polar solvent).
- NaCℓ (s) dissociates in an aqueous solution – the ions in the ionic crystal lattice separate.
- NaCℓ(s) H2O (ℓ) Na+ (aq) + Cℓ– (aq)
- A sodium chloride solution, NaCℓ (aq), is known as brine.
6.4 The chlor-alkali industry – industrial process
The chlor-alkali industry products can be produced by using a:
- membrane cell
- mercury cell
- diaphragm cell
| Membrane Cell | Diaphragm Cell | Mercury Cell | |
| Purity and yield of Cℓ2 (g) produced |
|
|
|
| Purity of NaOH (aq) produced | High |
| Low |
| Energy consumption (Affects costs) | Lowest | Relatively low | Very high |
| Environmental impact |
|
|
|
| Health risks |
|
|
|
NB:
- Very few companies still use mercury cells but many still use diaphragm cells. When new industrial sites are set up today, membrane cells are mostly used.
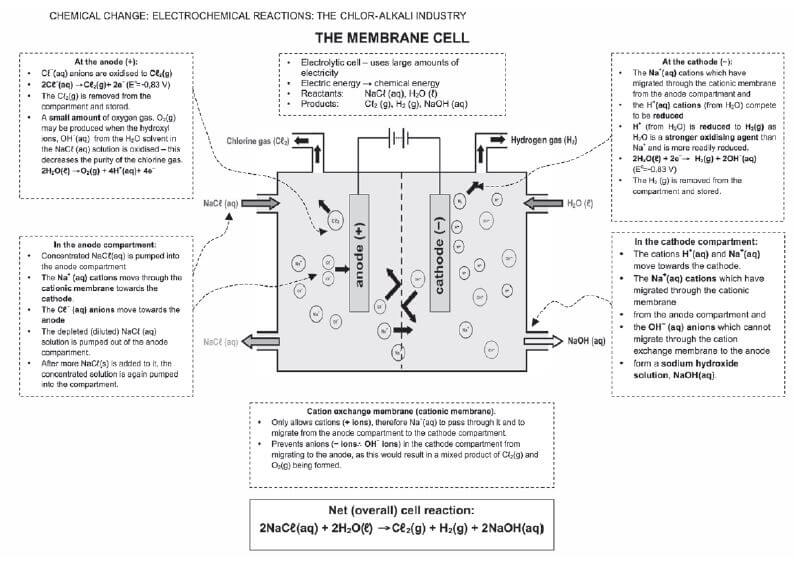
e.g. Worked example 1
1. Chlorine is produced industrially by electrolysis in an electrolytic cell and can be represented as follows:
2NaCℓ(aq) + 2H2O(ℓ) → H2(g) + Cℓ2(g) + 2NaOH(aq)
| I | 2H2O | + | 2e– | → | H2 | + | 2OH– |
| II | Cℓ2 | + | 2e– | → | 2Cℓ– | ||
| III | 2Cℓ– | → | Cℓ2 | + | 2e– | ||
| IV | H2 | + | 2OH– | → | 2H2O | + | 2e– |
The correct statement(s) is/are:
- I and III
- I only
- II and IV
- III only
2. Chlorine is a poisonous gas commonly used as a bleaching agent. Chlorine is produced in industry by _____________.
Solutions
- A
- Electrolysis of brine
Activity 1
The electrolysis of saturated sodium chloride can be illustrated as follows:
Hydrogen and chlorine bubble off at the electrodes.

- Give an equation to show how chlorine bubbles are formed at the electrode. (2)
- At which electrode are the chlorine bubbles formed? (1)
- At which electrode does hydrogen gas form? (1)
- What is the name of the product which leaves the cell? (1)
- Give TWO applications of the product formed in 2.4. (2)
- What purpose does the porous diaphragm serve? (2)
- The chlorine gas produced, dissolves in water to form chlorine water. Write down a balanced equation for the reaction that takes place. (2) [11]
Solutions
- 2Cℓ–(aq) → Cℓ2(g) + 2e– (2)
- Anode (1)
- Cathode (1)
- Sodium hydroxide (1)
- Making soap and detergents; paper; rayon and other fibres; dyeing textiles (any 2) (2)
- Stops chlorine passing through; helps to separate sodium hydroxide from NaCℓ(aq). (2)
- Cℓ2 + H2O → HCℓ + HOCℓ (2) [11]
e.g. Worked example 2
The diagram below shows a type of membrane cell used in the chlor-alkali industry.
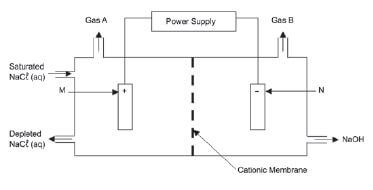
- Name the gases A and B
- Why is the membrane called a cationic membrane?
- Write down the half-reaction that takes place at electrode N.
- Apart from its use in household products, name ONE industrial use of chlorine.
- Explain why this electrolytic process cannot be done in one large container without a membrane.
Solutions
- A: Chlorine
B: Hydrogen - Allows only the positive ions (cations) to pass through it.
- 2H2O + 2e– → H2 + 2OH–
- Manufacture of PVC, paper, drugs, etc
Disinfectant for water - In a single pot the chlorine will react with water to form chlorine water/or the chlorine will react with the OH– ions to form bleach OR the products formed will be contaminated
Activity 2
The simplified diagram of a cell used in the chlor-alkali industry is shown below.

- Write down the CHEMICAL FORMULA of brine. (1)
- At which electrode, X or Y is chlorine gas formed? (1)
- Write down a half-reaction that explains the formation of hydrogen gas at one of the electrodes. (4)
- The purity of the sodium hydroxide produced in the chlor-alkali industry depends on the extent to which it is separated from the chlorine gas produced by this cell. Briefly describe how chlorine gas and sodium hydroxide are prevented from mixing in this cell. (2)
- Apart from the advantages and disadvantages of products produced, write down for this process:
5.1 ONE positive impact on humans. (1)
5.2 ONE negative impact on humans (1) [10]
Solutions
- NaCℓ (aq) (1)
- Y (1)
- 2H2O + 2e– →H2+2OH – (4)
- The membrane prevents chloride ions from moving to the cathode, only allows positive ions through. (2)
- 5.1 Job creation resulting in more people having a better life. (1)
5.2 Uses huge amounts of electricity resulting in load shedding.
OR
Chemical plant uses a lot of space that could have been used for housing/gardens, etc. (any one) (1)
ELECTROCHEMISTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
- Summary
- Key concepts: Definitions and terminology
- Electrochemical cells
- Electrolytic cells
- Application of electrolysis
- Voltaic (Galvanic) cells
- Cell notation
- Standard electrode potentials
- The standard hydrogen electrode
- The emf of an electrochemical cell
Electrochemistry
Electrochemistry refers to chemical reactions during which chemical energy is converted to electric energy, or electric energy is converted to chemical energy. During these chemical reactions oxidation and reduction take place. These are called redox reactions.
5.1 Summary
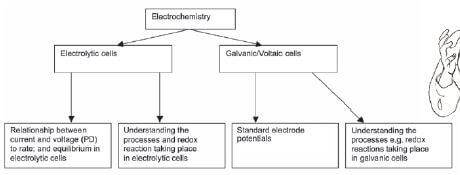
Redox (reduction - oxidation) reactions:
- Electron transfers take place.
- can be represented by two half-reactions:
- an oxidation half-reaction and
- a reduction half-reaction.
5.2 Key concepts: Definitions and terminology
NB
- Electrolysis is the chemical process in which electrical energy is converted to chemical energy.
- Electrolysis is the process where electrical energy is used to produce a chemical change.
Another name for a galvanic cell is a voltaic cell. A galvanic cell has self-sustaining electrode reactions.
Remember
You must remember these basic definitions for electrochemistry:
- Galvanic cells
- Electrolytic cells
- Oxidation
- Reduction
- Oxidising agent
- Reducing agent
- Anode
- Cathode
- Electrolyte
- Electrolysis
A galvanic cell is a cell in which chemical energy is converted into electrical energy.
An electrolytic cell is a cell in which electrical energy is converted into chemical energy.
NB: Remember
We define oxidation and reduction in terms of electron (e–) transfer
- RED CAT: REDuction – CAThode
- ANOX: ANode - OXidation
Oxidation | Reduction |
Oxidation is the loss of electrons by a substance (i.e. by an atom, a molecule or an ion). | Reduction is the gain of electrons by a substance (i.e. by an atom, a molecule or an ion). |
Learn: LEO for Loss of Electrons is Oxidation. | Learn: GER for Gain of Electrons is Reduction. (because gaining electrons is gaining minuses, so reducing) |
A substance that is oxidised (i.e. loses electrons) is called a reducing agent. | A substance that is reduced (i.e. gains electrons) is called an oxidising agent. |
The oxidation number of a compound that is oxidised, increases (becomes less negative, or becomes more positive). | The oxidation number of a compound that is reduced, decreases (becomes more negative, or becomes less positive). |
The anode (–) is the electrode where oxidation (+) takes place
The cathode (+) is the electrode where reduction (–) takes place.
The electrodes:
- conduct electricity
- are placed in electrolytes which are solutions consisting of ions.
The electrolyte is the solution/liquid/dissolved substance that conducts electricity through the movement of ions.
Activity 1
Give one word for the following statements:
- The chemical process when an electric current is passed through an ionic compound in solution or in molten state. (1)
- An ionic solution that conducts electricity. (1)
- The reactant that donates electrons during a redox reaction. (1)
- The electrode in an electrochemical cell where reduction takes place. (1)
- TRUE OR FALSE? The reactions C(s) + O2(g) → CO2(g) and 2KCℓO3(s) → 2KCℓ(s) + 3O2(g) are examples of redox reactions. (1)
- The electrode in an electrochemical cell where oxidation occurs. (1)
- A substance that shows a decrease in oxidation number during chemical reactions. (1)
- Which of the following substances can be used as an electrolyte?
- Mercury
- Molten copper
- Sugar dissolved in distilled water
- D. Table salt dissolved in distilled water. (1) [8]
Solutions
1. Electrolysis✓ (1)
2. Electrolyte✓ (1)
3. Reducing agent✓ (1)
4. Cathode✓ (1)
5. TRUE statement✓ (1)
6. Anode✓ (1)
7. Oxidising agent✓ (1)
8. D✓ (1) [8]
5.3 Electrochemical cells
Electrochemical cells allow conversion between electrical and chemical energy.
There are two types of electrochemical cells:
- Galvanic (voltaic) cells
- Electrolytic cells.
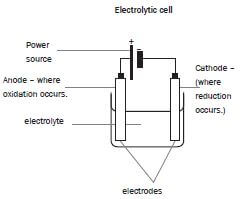 | 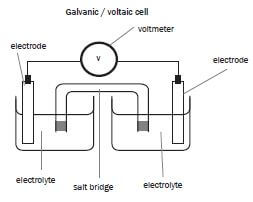 |
|
|
Activity 2
Give ONE word for the following statements:
- The electrode in a galvanic cell at which reduction occurs. (1)
- Anode in an electrolytic cell. (1)
- The type of electrochemical cell in which electrical energy is converted to chemical energy. (1)
- TRUE or FALSE? An electrolytic cell converts mechanical energy to electrical energy. (1)
- Which ONE of the following half-reactions occurs at the cathode during the electrolysis of an aqueous CuCℓ2 solution?
- Cℓ2 + 2e– → 2Cℓ–
- Cu+ + e– → Cu
- 2Cℓ– → Cℓ2 + 2e–
- Cu2+ + 2e– → Cu (2)
- The gain of electrons by a substance in a chemical reaction is known as …
- Oxidation
- Reduction
- Electrolysis
- D. Oxidation and reduction (2) [8]
Solutions
- Cathode✓ (1)
- Positive electrode✓ (1)
- Electrolytic✓ (1)
- This statement is FALSE. This cell converts electrical energy to chemical energy.✓ (1)
- D ✓✓ (2)
- B ✓✓ (2) [8]
5.4 Electrolytic cells
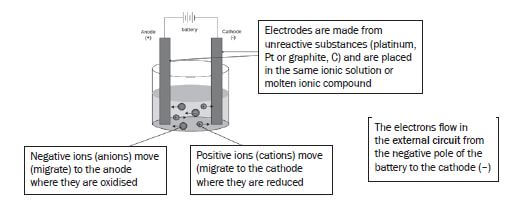
a).Electrolysis of molten ionic compounds:
- The negative ions migrate (move) to the anode where they are oxidised.
- The positive ions migrate to the cathode where they are reduced.
b). Electrolysis of ionic solutions:
The anions (negative ions) from the ionic compound and the hydroxyl ions (OH–) from the water migrate to the anode (positive electrode). The anions from the ionic compound compete with the hydroxide ions (OH–) from the solution, to be oxidised.
Rules:
If the ionic compound contains:
- halide ions (Cℓ–; Br– or I–), the halide ion will be oxidised and NOT the hydroxide ion. The product will therefore be the corresponding halogen.
- sulphate (SO42–) or nitrate (NO3–) ions, the hydroxide ion (OH–) will be oxidised to produce oxygen, O2(g).
The cations (positive ions) from the ionic compound and the H+ ions from the water migrate to the cathode (negative electrode).
The cations from the ionic compound compete with the H+ ions to be reduced.
Rules:
If the ionic compound contains:
- cations of metals with a positive electrode potential, these cations will be reduced to form the corresponding metal e.g. Cu. See the table on electrode potentials provided in introduction to this book.
- cations of metals with a negative electrode potential (e.g. Zn2+), the H+ ions will be reduced to form hydrogen, H2(g). See the table on electrode potentials provided in this book.
NB
Remember to indicate the charge on the ion
e.g. Worked example 1
- Explain how the electrolysis of a copper (II) chloride solution CuCℓ2(aq) takes place.
Solution
- The anions Cℓ– and OH– migrate to the positive anode. The Cℓ– ions are oxidised.
oxidation half-reaction: 2Cℓ–(aq) → Cℓ2(g) + 2e–
The cations (Cu2+ and H+) migrate to the cathode. The Cu2+ ions are reduced.
reduction half-reaction: Cu2+(aq) + 2e– → Cu(s)
The net reaction: Cu2+(aq) + 2Cℓ–(aq) → Cu(s) + Cℓ2(g)
The solution is initially blue due to the presence of the Cu2+ ions, but as they are reduced to Cu(s), the solution turns colourless and red-brown Cu(s) is deposited on the cathode.
e.g. Worked example 2
Predict the products of the electrolysis of a copper (II) sulphate solution CuSO4(aq)
Solution
2.
- At the anode: O2(g)
- At the cathode: Cu(s)
e.g. Worked example 3
Predict the products of the electrolysis of a zinc chloride solution ZnCℓ2(aq)
Solution
3.
- At the anode: Cℓ2(g)
- At the cathode: Zn(s)
c). Electrolysis of water:
Water is a weak electrolyte (weak electric conductor). A small amount of dilute sulphuric acid (H2SO4) is added to water to increase its conductivity. When an electric current is passed through the acidified water, oxidation and reduction reactions take place.
At the anode:
oxidation half-reaction: 2H2O → O2(g) + 4H+ + 4e– E0 = +1,23 V
At the cathode:
reduction half-reaction: 2H+ + 2e– → H2(g) E0 = 0,00 V
The net reaction: 2H2O(ℓ) → 2H2(g) + O2(g)
5.5 Application of electrolysis
- Electroplating
- Production of chemicals e.g. chlorine gas, hydrogen gas and sodium hydroxide (membrane cell, chlor-alkali industry)
- Extraction of metals e.g. aluminium
- Refining (purifying) of metals e.g. copper.
a). Electroplating
Electroplating is the process of putting a metallic coating on an object using electrolytic reactions.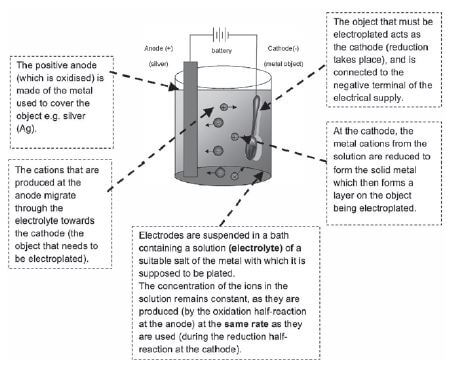
Electroplating is used to protect metals that oxidise easily, by covering them with a thin layer of a metal that does not oxidise easily e.g. chromium, silver or gold. A relatively cheap metal is covered by an expensive metal. Silver is used to cover cutlery — it is too expensive to make a spoon of pure silver, and it is too weak to use — while chromium can be used to cover car parts like bumpers.
e.g. Worked example 4
An attractive silver appearance can be created by electroplating artefacts (objects) made from cheaper metals, such as nickel, with silver. The simplified diagram here represents an arrangement that can be used to electroplate a nickel artefact with silver.
- Which electrode (cathode or anode) does the nickel artefact represent?
- Name the metal represented by electrode Y.
- Write down the half-reaction responsible for the change that occurs at the surface of the artefact.
- Give a reason why the concentration of the electrolyte remains constant during electroplating.
- In industry some plastic articles are sometimes electroplated. Explain why plastic must be coated with graphite before electroplating.
- Give a reason why, from a business point of view, it is not advisable to plate platinum with silver.
Solutions
- Cathode
- Silver
- Ag+ + e– → Ag
- The rate of oxidation of silver at the anode is equal to the rate of reduction of silver ions at the cathode.
- Plastic is a non-conductor. It must be covered with a conducting layer so that it can act as the cathode. Graphite is a conductor.
- Platinum is expensive and is more durable than other metals. You would normally electroplate a cheap metal with an expensive, durable metal and not the other way round.
Activity 3
- TRUE or FALSE? During electroplating of a steel teaspoon with silver, the teaspoon is the cathode and the electrolyte is a solution of any soluble compound [2]
Solution
- FALSE. ✓… the electrolyte is a solution of a soluble silver compound. ✓ [2]
b) Refining of copper
Copper which is mined is impure and the copper ore can be refined as follows by means of electrolysis:

e.g. Worked example 5
Impure copper can be purified by the process of electrolysis. The simplified diagram represents an electrolytic cell used to purify copper.
- Define the term electrolysis.
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer.
- Write down the half-reactions that take place at electrodes P and Q.
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process.
- Explain why the concentration of the copper (II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum.
- Why is the sludge of economic importance?
Solutions
- Electrolysis is a process during which electrical energy is converted to chemical energy. It is the process in which electricity is used to bring about a chemical change / decompose / break compounds into components.
- P: P is the positive electrode / anode. The impure Cu is oxidised at the positive electrode / anode.
- P: Anode: Cu(s) → Cu2+(aq) + 2e– oxidation impure
Q:Cathode: Cu2+(aq) + 2e– → Cu(s) reduction pure - Platinum and silver are both weaker reducing agents than copper and will not be oxidised to form ions.
- The rate at which copper is oxidised (at the anode) is equal to the rate at which copper ions are reduced (at the cathode).
- Silver and platinum are valuable and expensive metals and can therefore be sold at a profit.
Activity 4
- Refer to the diagram in the worked example above. One of the electrodes consists of impure copper and the other one of pure copper.
- What type of power source is used to drive the reaction in this cell? Write down only AC or DC. (1)
- Give a reason why the copper(II) sulphate is dissolved in water before it is used in this cell. (1)
When an electric current passes through the solution, electrode P becomes coated with copper. - Is electrode P the cathode or the anode? Support your answer by writing the half-reaction that takes place at electrode P. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
It is found that the impure copper plate contains platinum. The platinum forms a residue at the bottom of the container during electrolysis. - Refer to the relative strengths of reducing agents to explain why platinum forms a residue at the bottom of the container. (2)
- How will the concentration of the copper(II) sulphate solution change during electrolysis? Write down only INCREASES, DECREASES or REMAINS THE SAME. (3) [11]
Solutions
- DC ✓ (1)
- Free ions needed to conduct electricity ✓ (1)
- Cathode. ✓ Cu2+ + 2e– → Cu ✓ (2)
- Cu → Cu2+ + 2e– ✓✓(2)
- Pt is a weaker reducing agent than Cu ✓and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent than Pt and will be oxidised - Remains the same.✓ The rate at which Cu is oxidised at the anode ✓ equals the rate at which Cu2+(aq) is reduced at the cathode ✓ (3) [11]
c) The recovery or extraction of aluminium metal from bauxite
NB: A very important application of electrolysis is the recovery of aluminium. South Africa imports bauxite, an aluminium ore.
Aluminium:
- is one of the most abundant metals on earth, yet it is expensive – largely because of the amount of electricity needed to extract it.
- has the following properties: a low density; the ability to resist corrosion; is very ductile; can be rolled out in thin layers; is lightweight and is a good electrical conductor.
The process involves:
- Aluminium oxide (Aℓ2O3) is extracted from the bauxite and it is then heated.
- The melting point of Aℓ2O3 is higher than 2 000°C. Cryolite (Na3AℓF6) is added to the ore before it is heated. Cryolite does not lower the melting point of Aℓ2O3 but dissolves it. Aℓ2O3 dissolved in a molten cryolite can be electrolysed easily. This means that less electricity is required to extract the aluminium, thereby decreasing the extraction cost.
- The ions, Aℓ3+ and O2–, are formed during the extraction.
- The mass of the anode therefore gradually decreases. The cost of replacing the anode when it is depleted adds to the high cost of the aluminium extraction.
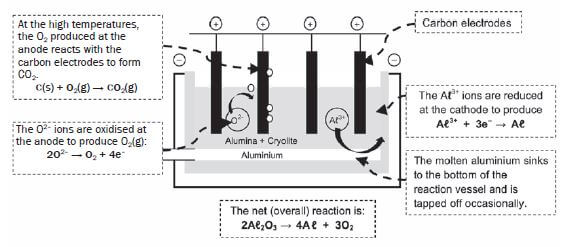
- The ecological impact of Aℓ extraction: Loss of landscape due to the size of the chemical plant needed; disposal of red mud (iron (III) oxide) formed during extraction of aluminium oxide from bauxite, into rivers and lagoons and into ground water.
- Environmental impact of Aℓ extraction: Carbon dioxide from the burning of the anodes contributes to the greenhouse effect, causing global warming. Fluorine and its compounds lost from the cryolite during the electrolysis process are poisonous. Chemicals in the red mud dams drain into the soil and contaminate groundwater. Pollution caused by power generation (for electrolytic process) using coal fired plants leads to acid rain and adds to the greenhouse effect. Noise pollution from the extraction plant.
Activity 5
Give ONE word for the following phrase:
- The main ore from which aluminium is extracted. (1)
- The name of the chemical substance in which Aℓ2O3 is dissolved to lower its melting point during the industrial extraction of aluminium. (1) [2]
Solutions
- Bauxite ✓
- Cryolite ✓ [2]
Activity 6
- In an aluminium smelter, aluminium metal is extracted from bauxite, a hydrated aluminium oxide, via an electrolytic process.
1.1 Write down the energy conversion that takes place in an electrolytic cell. (2)
1.2 Write down the equation for the half reaction responsible for the formation of aluminium metal in a smelter. (2)
1.3 Explain in terms of the relative strength of oxidizing agents why the electrolytic production of aluminum requires more electrical energy than that of iron or copper. (2)
1.4 Name TWO advantages that the use of aluminium has over that of iron. (2) - A huge aluminium smelter is planned for Coega in the Eastern Cape. When operational, it will consume 1350 MW of electricity, or 4% of the nation’s total electrical energy. It is estimated that 5200 jobs will be created at the peak of construction. About 1000 workers will be employed on a full-time permanent basis, and between 200 and 300 full-time subcontractors will also be directly associated with the smelter. (Source: www.engineeringnews.co.za; www.groundwork.org.za).
2.1 Taking the present South African socio-economic realities into account, give ONE reason why the aluminium smelter should:- Not be built (1)
- Be built (1)
2.2 Give ONE reason why environmental activists oppose the construction of the smelter. (1) [11]
Solutions
1.1 Electrical energy → chemical energy ✓✓ (2)
1.2 Aℓ3+ + 3e– → Aℓ ✓✓ (2)
1.3 Aluminium has a lower reduction potential (–1,66 V) ✓/ Weaker oxidizing agent compared to that of iron (–0,44 V) [and copper (+0,34 V)]. The aluminium ions therefore require a large amount of energy to be reduced/ will reduce more difficultly than iron (and copper). ✓ (2)
1.4 It is much lighter for the same strength (or stronger for the same mass). ✓ It is corrosion free.✓ (2)
2.1
- It will consume huge amount of electricity. ✓/ (1) Will cause power failures ✓ (any 2)
- It will create jobs ✓/Create foreign investment. ✓ (1) Contribute to GDP ✓ (any 3)
2.2 The production of the large amount of electricity used ✓ enhances the greenhouse effect (or climate change) ✓ OR The process is responsible for toxic fluoride waste OR pollution. (any one) (1) [11]
5.6 Voltaic (Galvanic) cells
Remember:
A Galvanic or voltaic cell is a cell in which chemical energy is converted to electrical energy spontaneously. We therefore use a chemical reaction to produce electricity. E.g. standard AA / penlight batteries.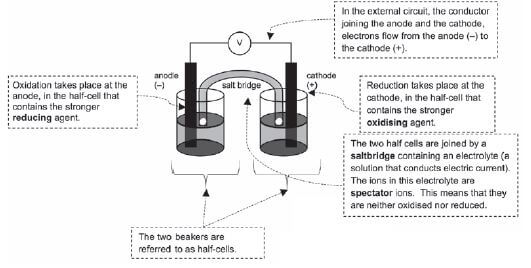
Consider a copper-zinc cell:
A strip of zinc metal is placed in a zinc ion solution. A strip of copper is placed in a separate beaker in an aqueous copper (II) ion solution. These solutions are the electrolytes. As they contain ions which dissociated when the salts were dissolved in water, they are good electric conductors.
- The half reactions that occur in each half cell:
Zn(s) → Zn2+ + 2e– Cu2+ + 2e– → Cu(s)
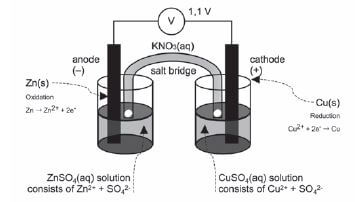
REMEMBER
- When redox half-reactions are to be written, the correct single arrow should be used.
| Zn half-cell | Cu half-cell |
| Oxidation half-reaction at the anode which is always the negative electrode (e– are given off) | Reduction half-reaction at the cathode which Is always the positive electrode. |
The stronger reducing agent is oxidised (gives off electrons) | The stronger oxidising agent is reduced (receives electrons) |
Zn(s) → Zn2+ + 2e– (read from right to left) | Cu2+ + 2e– → Cu(s) (read from left to right) |
| Zn is the stronger reducing agent | Cu2+ is the stronger oxidising agent |
The anode always decreases in mass if the reducing agent is a solid (The Zn rod is corroding). | The cathode always increases in mass if the reduced product is a solid (copper deposits on rod). |
Net cell reaction:
| |
NB
- In redox reactions, most learners struggle to understand the difference between the name of the process, such as oxidation and the substance, for example the reducing agent.
- Be very careful not to write reduction agent instead write reducing agent.
- Be able to clearly distinguish between the oxidising and the reducing agent.
a). Salt bridge – part of electrochemical cell (usually a tube) containing electrolyte providing electrical contact between two solution.
The salt bridge:
- Completes the circuit.
- Keeps the two electrolytes in the two half-cells separate so that they do not mix.
- Allows movement of ions between the electrolytes, so as to ensure electrical neutrality i.e. it acts as an ion exchanger.
- Contains a saturated salt solution (not 1 mol⋅dm–3) of either KNO3 or KCℓ.
- The electrolyte in the salt bridge must contain ions which are weak reducing agents and weak oxidising agents. This will ensure that these ions are not oxidised or reduced, but act as spectator ions.
NB: Potassium chloride is not suitable for a silver half-cell, because AgCℓ is formed and this compound is insoluble, thus a precipitate will form
b). Electrolyte:
- The salt of the compounds need to be soluble.
- Suitable soluble salts of zinc and copper are zinc nitrate and copper nitrate.
- The electrolyte solutions in this cell is Zn(NO3)2(aq) and Cu(NO3)2(aq).
- As the Zn(s) is oxidised to Zn2+(aq), the concentration of the Zn2+ ions increases in the diagram above. This means that the Zn half-cell will start building up a positive charge. As the electrons will only move away from a negative potential, the function of the cell would become impaired.
- In the same way as the Cu2+ ions are reduced to Cu(s), the concentration of the Cu2+ ions decreases. This leads to the copper half-cell becoming less positive. The electrons are therefore not attracted to the cathode as strongly and the function of the cell would become impaired (weaker).
- The migration of the NO3– anions in the salt bridge to the anode (the Zn half-cell) and of the K+ ions in the salt bridge to the cathode (the Cu half-cell), cancel this build-up of undesired charge and maintain the cell’s electric neutrality. The cell therefore continues to function properly.
NB: The nitrate salts of ionic compounds are very suitable as an electrolyte, since the salts are soluble in water.
c). Electron current:
- Electrons always flow from the anode, through the external circuit, to the cathode.
- In the Cu/Zn cell, electrons therefore flow from the zinc (Zn) to the copper (Cu).
5.7 Cell notation
The structure of the galvanic cell may also be represented in symbols.
Rules:
- Always start with the anode on the left and end with the cathode on the right.
- Use a / to separate the anode or cathode from its electrolyte.
- Represent the salt bridge with the symbol //.
- Inert electrodes (usually Pt or C) and phases are usually included:
Zn(s) / Zn2+(aq) // Cu2+(aq) / Cu(s)
electrode / electrolyte // electrolyte / electrode
anode (–) salt bridge cathode (+) - e.g. Pt/reducing agent/oxidised species//oxidising agent/reduced species/Pt
Pt / Cℓ–(aq) / Cℓ2(g) // F2(g) / F–(aq) / Pt
Activity 7
Give ONE word for the following phrase:
- The electrode in a galvanic cell at which reduction occurs. (1)
- The component of a galvanic cell that allows for the movement of ions between the half-cells. (1)
- Which statement is CORRECT for a Zn-Cu galvanic cell that operates under standard conditions? (Standard Conditions are defined in the tables at the beginning of this book)
- The concentration of the Zn2+ ions in the zinc half-cell gradually decreases.
- The concentration of the Cu2+ ions in the copper half-cell gradually increases.
- Negative ions migrate from the zinc half-cell to the copper half-cell.
- The intensity of the colour of the electrolyte in the copper half-cell gradually decreases. (2)
- The reactions below occur in two different electrochemical cells X and Y.
Cell X: CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Cell Y: Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Which ONE of the following correctly describes the substance that forms at the CATHODE of each of these cells?
Cell X | Cell Y | |
| A | Cℓ2(g) | Cu(s) |
| B | Cu(s) | Cu(s) |
| C | Cℓ2(g) | ZnSO4(aq) |
| D | Cu(s) | ZnSO4(aq) |
5. Which one of the following statements regarding a copper-silver galvanic cell is TRUE?
- Silver is formed at the anode
- Copper is formed at the anode
- Silver is formed at the cathode
- Copper is formed at the cathode (2) [8]
Solutions
1. Cathode ✓ (1)
2. Salt bridge ✓ (1)
3. D ✓✓ (2)
4. B ✓✓ (2)
5. C ✓✓ (2) [8]
5.8 Standard electrode potentials
The Reactivity Series is a list of substances which are arranged in order of their ability to act as reducing agents or as oxidising agents.
The Table of Standard Reduction Potentials lists the standard electrode potentials (E0 values) for various compounds. There are two tables, they are similar, but the entries are arranged in opposite directions.
- The tables of Standard Reduction Potentials can be used to:
- identify oxidising and reducing agents
- write balanced redox reaction equations
- predict whether a redox reaction takes place spontaneously or not
- calculate the emf of a voltaic cell by using one of the following formulae (formulas):
NB
- You will notice that all ionic compounds, for example, H2SO4, HNO3 and HCℓ must be dissociated into ions, before we can use the information in the Table of Standard Reduction Potentials.
E0cell = E0cathode – E0anode OR E0cell = E0oxidising agent – E0reducing agent
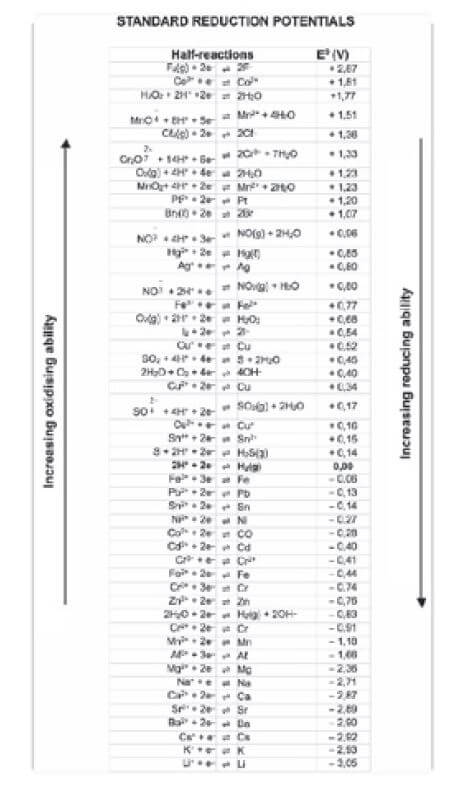
REMEMBER
- The formula must be written without any abbreviation in the subscript.
NB
- Further information is discussed on the Table of Standard Electrode Potentials (shown here so you know which table to look for). This table is given at the beginning of this book.
E.g. Stronger reducing agents displace weaker reducing agents, and remember to always start with the oxidation half-reaction. Please read that information now.
e.g. Worked example 6
A piece of zinc metal (Zn) is placed in a beaker containing a copper (II) sulphate solution and a piece of copper metal (Cu) is placed in a beaker containing a zinc (II) sulphate solution.
- Predict which redox reaction will take place spontaneously.
- Motivate your answer.
- Write the oxidation half-reaction, the reduction half-reaction and the net cell reaction for the spontaneous redox reaction.
Solutions
- Zn is oxidised to Zn2+ and Cu2+ is reduced to Cu. The redox reaction in the beaker containing the piece of zinc (Zn) and the copper (II) sulphate solution with Cu2+ ions is spontaneous.
- Zn is a stronger reducing agent than Cu (see reducing ability on table), i.e. Zn will be oxidised more readily than Cu and Cu2+ is a stronger oxidising agent than Zn2+ (see oxidising ability on table) i.e. Cu2+ will be reduced more readily than Zn2+. Therefore Zn will displace Cu from CuSO4(aq).
- oxidation half-reaction : Zn(s) → Zn2+(aq) + 2e–
reduction half-reaction: Cu2+(aq) + 2e– → Cu(s)
Overall or net ionic reaction is: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
e.g. Worked example 7
- Use the Table of Standard Reduction Potentials to determine whether Ag will spontaneously displace Zn2+ ions from a zinc sulphate (ZnSO4) solution. Motivate your answer.
- Write the oxidation half-reaction, the reduction half-reaction and the net ionic reaction which takes place when a piece of copper (Cu) is dipped in a silver nitrate (AgNO3) solution.
Solutions
- No, the reaction will not take place.
Ag is a weaker reducing agent than Zn, therefore it will not be able to displace Zn from the zinc sulphate solution. - oxidation half-reaction: Cu → Cu2+ + 2e–
reduction half-reaction: Ag+ + e– → Ag+
Multiply this reaction by 2, so that the number of electrons released by the copper atom equals the number of electrons gained.
2Ag+ + 2e– → 2Ag+
net ionic reaction: Cu + 2Ag+ → Cu2+ + 2Ag+
Activity 8
- Which one of the following solutions can be stored in an aluminium container?
(Use the Table of Standard Reduction Potentials.)- CuSO4(aq)
- ZnSO4(aq)
- NaCℓ(aq)
- Pb(NO3)2(aq) (2) [2]
Solution
1. C ✓✓[2]
Activity 9
The galvanic cell represented in the diagram consists of a Mg electrode dipped into a Mg(NO3)2 solution, and a Pb electrode dipped into a Pb(NO3)2 solution. Assume that the cell operates under standard conditions.

1. State TWO standard conditions under which this cell operates. (2)
2. Write down the half-reaction that takes place in half-cell A. (2)
3. Write down the cell notation for this cell. (2)
4. Calculate the emf of this cell. (3)
5. How will each of the following changes influence the value of the cell’s emf calculated in QUESTION 2.4? Write down only INCREASES, DECREASES or REMAINS THE SAME
5.1 An increase in [Mg2+(aq)] (1)
5.2 An increase in [Pb2+(aq)] (1)
6. In which direction, from half-cell A to B or from half-cell B to A, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer. (3) [14]
Solutions
1. Temperature: 298 K (25 °C) ✓✓(2)
2. Mg(s) → Mg2+(aq) +2e– ✓✓ (2)
3. Mg(s)/Mg2+(1 mol·dm–3) ✓// Pb2+(aq)(1 mol·dm–3)/Pb(s) ✓ (2)
4. E0cell = E0cathode – E0anode = - 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.
5.1 DECREASES ✓ (1)
5.2 INCREASES ✓ (1)
6.
- Half-cell A to half-cell B ✓
- Concentration of positive ions/cations ✓/ Pb2+ ions decreases in half-cell B. ✓
OR - Concentration of positive ions/cations/Mg2+ ions increases in half-cell A
- To prevent a build-up of positive ions in half-cell A and negative ions in half-cell B /for electrical neutrality.
- Positive ions migrate from/through the salt bridge (3) [14]
Activity 10
Learners conduct an investigation to determine which combination of two half-cells will provide the largest emf under standard conditions.
Three half-cells, represented as A, B and C in the table below, are available.
Half-cell A | Half-cell B | Half-cell C |
Mg/Mg2+ | Pb/Pb2+ | Aℓ/Aℓ3+ |
The learners set up galvanic cells using different combinations of the above half-cells.
- Write down the standard conditions under which these cells operate. (2)
- Write down the dependent variable in this investigation. If you can’t remember what the dependent (and independent) variables are, please see the introductory materials at the beginning of this book. (1)
- Use the Table of Standard Reduction Potentials to determine which one of the three half-cells (A, B or C) contains the:
3.1 Strongest reducing agent. (1)
3.2 strogest oxidizing agent. (1) - Without any calculation, write down the combination of two half-cells which will produce the highest emf. Write down only AB, BC or AC. (1)
- One group of learners set up a galvanic cell using half-cells A and B, as shown below. X is one of the components of the galvanic cell.

5.1 Write down the NAME or SYMBOL of the substance that will act as the anode in this cell. Give a reason for the answer. (2)
5.2 Calculate the initial emf of this cell. (3)
5.3 How will an increase in the concentration of the electrolyte in half-cell B affect the initial emf of this cell? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
5.4 Briefly explain how component X ensures electrical neutrality while the cell is functioning. (1) [13]
Solutions
- Temperature 25 °C /298 K ✓
Concentration of electrolytes = 1 mol·dm–3 ✓ (2) - emf/potential difference ✓ (1)
- 3.1 Half-cell A ✓ (1)
3.2 Half-cell B ✓ (1) - Combination AB ✓ (1)
- 5.1 Magnesium/Mg. 3Is oxidised/loses electrons/increase in oxidation number/stronger reducing agent ✓ (2)
5.2 E0cell = E0cathode – E0anode = – 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.3 Increases ✓ (1)
5.4 Allows for the migration of positive ions to the cathode half-cell ✓ (1)
OR
Allows for the migration of negative ions to the anode half-cell [13]
5.9 The standard hydrogen electrode
The hydrogen gas/hydronium ion electrode has been chosen as standard half-cell.
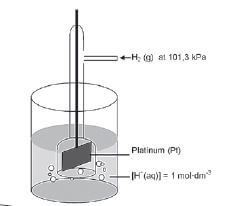

Activity 11
- Which is the the reference electrode in the diagram above? (1)
- TRUE or FALSE? A standard cell that has a negative emf cannot be used as a galvanic cell. (2)
- TRUE or FALSE? The standard conditions used to measure standard electrode potentials are: A temperature of 273 K, a concentration of 1 mol·dm–3, and a pressure of 101,3 kPa. (2)
- Which ONE of the following containers can be used to store an iron(II) sulphate solution?
- Aℓ
- Mg
- Ni
- Zn (2) [7]
Solutions
- Hydrogen half-cell ✓ (1)
- TRUE ✓ (2)
- FALSE. ✓ A temperature of 298 K (25 °C) — the question gives the temperature incorrectly ✓(2)
- C ✓ (2) [7]
NB
- A half-cell potential of 0,00 V has been assigned to the standard hydrogen electrode. The half-cell potential values are given on the right hand side of the table.
- To determine the standard electrode potential of the copper half-cell

- To determine the standard electrode potential of the zinc half-cell

5.10 The emf of an electrochemical cell
- A voltmeter connected to the zinc / copper cell has a reading of 1,1 V. It reads the difference between the electric potentials of the two separate electrodes.
- This reading of 1,1 V can also be referred to as the potential difference or cell potential or emf of the cell.
e.g. Worked example 8
- Using the Cu / Zn cell as an example:
E0cell = E0cathode – E0anode
+0,34 – (–0,76) = +0,34 + 0,76
+1,1 V
NB
- Always use the values exactly as they stand on the table; do not round off.
- The cell potential (voltage) does not depend on the surface area of the electrode.
- The bigger the surface area of the electrode, the bigger the current produced by the cell.
- In general the cell potential increases slightly with an increase in temperature.
- The reaction in the cell reaches equilibrium when the cell is completely discharged (flat or dead). This usually means that the concentration of the cations in the reduction half-cell (i.e. at the cathode) has decreased to zero.
Using the Table of Standard Reduction Potentials to predict whether a redox reaction will be spontaneous or not:
- Assume that the reaction will take place as suggested in the question.
- Identify the reducing agent and the oxidising agent according to the given equation.
- Calculate the cell potential for this reaction.
- If the cell potential is positive, the reaction will be spontaneous i.e. it will take place without the addition of any other form of energy. If it is negative, the reaction will not occur spontaneously.
- If the two half reactions are very close to each other on the Table of Standard Reduction Potentials, heating the reaction mixture may supply enough additional energy for the reaction to take place.
Steps
- Step 1. Use the Reduction Potentials to determine whether a reaction will take place spontaneously.
- Step 2. If the calculated cell potential is a positive value the redox reaction will take place spontaneously.
NB:
H2S → S + 2H+ + 2e–
OR
S + 2H+ + 2e– ← H2S- is the only correct way of writing a half-reaction
- Note the direction of the single arrow changes, depending on whether the reaction is an oxidation or reduction half reaction.
- Please do NOT include the double arrow in the half reaction.
- Always remember to include the charges on the ions in the half-cell reactions.
NB
When you have to explain the relative strength of oxidizing and reducing agents, write the explanations as follows:
- Cu is a stronger oxidising agent than Mg. Do NOT state the position of a substance in terms of its position in the Reduction Table (e.g. Cu is above Mg)
- Do NOT state the relative strength in terms of relative reactivity only, for example, Mg is more reactive than Cu.
Your correct answer would read as follows:
- Mg is a stronger reducing agent than Cu and therefore Mg will be able to reduce Cu2+ ions to Cu.
OR
Explain the relative strength of the oxidizing and reducing agents in terms of their relative strength as electron acceptor and donors.
- Mg is a stronger reducing agent than Cu because Cu has a stronger tendency to accept electrons than Mg. OR: Mg has a stronger tendency to donate electrons to Cu than Cu has to donate electrons to Mg.
e.g. Worked example 9
Batteries consist of one or more galvanic cells. A galvanic cell is a combination of two half-cells. John wants to determine which one of Options A or B, shown below, can be used to assemble a galvanic cell with the highest potential difference.
| Option | Combination of half-cells |
| A | Ag(s) in AgNO3(aq) & Ni(s) in Ni(NO3)2(aq) |
| B | Mg(s) in Mg(NO3)2(aq) & Ag(s) in AgNO3(aq) |
- Draw a fully labelled diagram of the galvanic cell that John can use to measure the potential difference for the cell in Option B. Use a positive (+) and negative (–) sign to indicate the positive and negative electrodes respectively.
- Write down the oxidation and reduction half reactions as well as a balanced chemical equation, excluding spectator ions, for the net (overall) cell reaction for the galvanic cell in Option B.
- Calculate the initial potential difference that can be obtained under standard conditions for the galvanic cell in Option B.
- State TWO standard conditions that John must adhere to during the experiment, to ensure that the measured potential difference is the same as the calculated potential difference.
- Write down the cell notation for the galvanic cell in Option A.
- Without doing any calculations, determine which one of Option A or Option B should result in the galvanic cell with the highest potential difference. Refer to the relative strengths of the two reducing agents, as well as the two oxidising agents involved, to explain your answer.
- How will each of the following changes influence the value of the cell’s emf calculated in Question 3?
7.1 An increase in [Mg2+(aq)].
7.2 An increase in [Ag+(aq)]. - In which direction, from half-cell Mg2+ / Mg to Ag + / Ag, or from half-cell Ag + / Ag to Mg2+ / Mg, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer.
- Give two functions of the salt bridge.
- Name the cathode and the anode for the galvanic cell in option B.
- Which electrode will show an increase in mass for the galvanic cell in option B?
- Distilled water is added to the Ag+ solution for the galvanic cell in Option B. How will the emf of the cell be influenced? Explain your answer.
- Consider the galvanic cell in Option B. If 0,6 moles of electrons flow from the anode to the cathode, what will the decrease in mass of the anode be?
- A 2 V bulb is connected to the cell in option B instead of the voltmeter. Will the bulb light up? Justify your answer.
- Consider the galvanic cell in Option B. It is observed that after a while, the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell.
- Give two uses of galvanic cells.
Solutions
-

- oxidation: Mg(s) → Mg2+(aq) + 2e–
reduction: Ag+(aq) + e– → Ag(s)
net reaction: Mg(s) + 2Ag+(aq) → Mg2+(aq) + 2 Ag(s) - E0cell = E0cathode – E0anode = 0,80 – (–2,36) = 3,16 V
- Ensure a temperature of 25 °C and Mg2+, Ni2+ and Ag+ solutions of concentration 1 mol·dm3.
- Ni(s) / Ni2+(aq) (1 mol·dm–3) // Ag(s)+(aq) (1 mol·dm–3) /Ag(s)
- Option B. The reaction leading to the highest emf (or potential difference) will be between the strongest reducing agent (Mg) and the strongest oxidising agent (Ag+).
- An increase in the rate of the forward reaction will increase the emf of a galvanic cell.
7.1 decreases; the reverse reaction is favoured
7.2 increases; the forward reaction is favoured - Half-cell Mg2+ / Mg to Ag + / Ag
Concentration of positive ions or cations; [Ag+] ions decreases in half-cell Ag + / Ag. OR
Concentration of positive ions or cations; [Mg2+] ions increases in half-cell Mg2+ / Mg.
To prevent a build-up of positive ions in half-cell Mg2+ / Mg and negative ions in half-cell Ag+ / Ag.
For electrical neutrality, positive ions migrate from/through the salt bridge to the cathode. - Any two: It completes the circuit. It ensures electrical neutrality by allowing the migration of ions through the salt bridge. It separates the electrolytes.
- cathode = Ag anode = Mg
- cathode = Ag
- By adding water to the solution, the [Ag+] will decrease. Thus the reverse reaction is favoured and emf decreases.
- Mg(s) → Mg2+(aq) + 2e– (anode)
From the balanced equation 1 mol Mg → 2 mol e–
thus 0,3 mol Mg → 0,6 mol e–
n = m/M
molar mass (M) of Mg = 24 g·mol–1
m = n × M
m = 0,3 × 24 = 7,2 g
mass loss is 7,2 g - The bulb will light up, as the emf of the cell is 3,16 V which is more than the required 2 V.
- While the cell is in operation, the concentration of the cations in the reduction half-cell Ag+(aq) decreases. At the same time, the concentration of the cations in the oxidation half-cell Mg2+(aq) increases. The result is a gradual decrease in the cell potential until there is no further change in the concentration and equilibrium is reached, and the cell potential will be zero.
- Any two: Torch cells, car batteries, batteries in toys and small appliances like remote controls.
e.g. Worked example 10
Rusting is an unwanted redox reaction. Iron rusts when exposed to oxygen and moisture. The unbalanced ionic equation for one reaction that occurs during rusting is represented below.
Fe(s) + O2(g) + H2O(ℓ) → Fe2+(aq) + OH–(aq)
Use the Table of Standard Reduction Potentials to answer the following questions for this reaction:
- Write down the oxidation and the reduction half-reactions.
- Write down the NAME of the substance that is reduced.
- Perform a calculation to verify that this reaction is spontaneous.
Solutions
- oxidation: Fe → Fe2+ + 2e–
reduction: 2H2O + O2 + 4e– → 4OH– - oxygen
- oxidation: Fe → Fe2+ + 2e– E0anode = –0,44 V
reduction: 2H2O + O2 + 4e– → 4OH– E0cathode = 0,40 V
E0cell = E0cathode – E0anode = 0,4 – (–0,44) = 0,84 V
Because E0cell is positive, the reaction is spontaneous.
e.g. Worked example 11
Magnesium is used to protect underground iron pipes against rusting (cathodic protection). The diagram here shows an iron pipe connected to a magnesium bar.
- Use the Table of Standard Reduction Potentials to explain why magnesium can be used to protect an iron pipe against rusting.
- The iron pipe in contact with the magnesium bar forms an electrochemical cell. What serves as the salt bridge of this cell?
- Give a reason why the magnesium bar must be replaced after some time.
- Write down a half-reaction to support your answer to Question 3.3.
- Name TWO other methods that can be used to protect iron pipes against rust.
- State ONE advantage and ONE disadvantage of using plastic pipes instead of iron pipes.
Solutions
- Mg is a stronger reducing agent (than Fe) and will be oxidised more readily than Fe which is a weaker reducing agent.
- The salts which are dissolved in the moist soil.
- Mg is continuously oxidised to Mg2+ and therefore its mass decreases i.e. it is used up.
- Mg → Mg2+ + 2e–
- Any two: Paint; electroplating e.g. galvanising; oil or waterproofing; plastic coating.
- Advantages: Plastic is cheaper; plastic does not rust.
Disadvantages: Plastic not biodegradable; plastic is not as strong as iron.
Activity 12
Give ONE word for the following phrase:
- The process taking place in a cell when an electric current passing through its electrolyte, results in chemical reactions at its electrodes. (1)
- TRUE or FALSE? A battery labelled as 3 000 mA·h can deliver a current of 500 mA for 6 hours. (2) [3]
Solutions
1. Electrolysis ✓ (1)
2. True. ✓✓ (2) [3]
Activity 13
- The most common filling for tooth cavities is ‘dental amalgam’ – a solid solution of tin and silver in mercury. If you bite on a piece of aluminium foil that is in contact with a dental filling in your mouth, you may feel a painful sensation because
- the aluminium foil is hard.
- a temporary galvanic cell has been set up whilst the aluminium and filling are in contact.
- electrons are being transferred to the aluminium.
- a temporary electrolytic cell has been set up whilst the aluminium and filling are in contact. (2)
- Which one of the following can be classified as a redox reaction?
- NH3(g)+ HNO3(g) →NH4NO3(s)
- FeS(aq)+ 2HCℓ(aq) → FeCℓ2(aq) + H2S(g)
- Aℓ(s) + Cℓ2(g) →AℓCℓ3(s)
- Mg(NO3)2(s) → Mg2+(aq) + 2NO3–(aq) [with H2O catalyst] (2)
- A silver (Ag) spoon is left in a beaker containing copper nitrate, Cu(NO3)2, solution. What will be observed after some time?
- The spoon will be covered with a thin layer of copper.
- The nitrate will form NO2 gas and copper will form in the beaker.
- The spoon will start to erode and its mass will decrease.
- There will be no reaction.
Solutions
1. B. ✓✓ (2)
2. C. ✓✓ (2)
3. D. ✓✓ (2) [6]
Activity 14
- A group of learners set up an electrochemical cell using lead and copper half cells.
1.1 Which ONE of copper or lead will be the negative electrode? Give a reason for your answer. (2)
1.2 Use the Table of Standard Reduction Potentials and write down the reduction half-reaction that will take place in this cell. (2)
1.3 A 2V bulb is connected to the cell. Will the bulb light up? Justify your answer with a calculation. (5)
1.4 A voltmeter is now connected to the cell instead of the bulb. It is observed that after a while the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell. (3) - A cell such as the one described above is not much useful. However, the principle is used in batteries for cars, torches, computers, et cetera. These batteries are called secondary cells. One such battery is the mercury cell. The half reactions occurring in this cell are shown below.
Zn(s) + 2OH–(aq) → ZnO(s) + H2O + 2e–……… (i)
HgO(s) + H2O + 2e– → Hg(ℓ) + 2OH–(aq)….….. (ii)
2.1 Write down the overall cell reaction. (3)
2.2 Why does the use of this battery pose an environmental hazard? (1) [16]
Solutions
1.1 Lead. ✓ Stronger reducing agent ✓ OR Is oxidised preferably (2)
1.2 Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
1.3 E0cell = E0cathode − E0anode ✓ = 0,34– (–0,13) ✓ =0,47V✓ (5) Bulb will not light, ✓ energy from cell not sufficient ✓ OR emf of cell is much less than 2 V needed for the bulb ✓✓
1.4. While the cell is in operation, the concentration of the reactants (Cu2+(aq)) decreases. ✓ At the same time the concentration of the products (Pb2+(aq)) increases. ✓ The result is a gradual decrease in the cell potential until there is no further change in concentration and equilibrium is reached where the cell potential will be zero. ✓ (3)
2.1 Zn(s) + HgO(s) → ZnO(s) + Hg(ℓ) ✓✓✓ (3)
2.2 Mercury is poisonous ✓ (1) [16]
Activity 15
The discovery of electrochemical cells has revolutionised our way of life.
The diagram below represents an electrochemical cell.
- Name the type of electrochemical cell that converts chemical energy to electrical energy. (1)
- If the electrochemical cell is set up as illustrated, there will be no reading on the voltmeter. Give a reason for this observation.(1)
- Write down the value of the standard emf of the electrochemical cell when it is functioning. (1)
- Write down the voltmeter reading when the net cell reaction in the above electrochemical cell reaches equilibrium. (1)
- Write down the equation for the reaction that occurs at the anode. (1)
- Another electrochemical cell is set up under standard conditions by replacing the standard hydrogen half-cell with a standard magnesium half-cell.
6.1 Which electrode will undergo a decrease in mass? Give a reason for your answer. (2)
6.2 Calculate the initial emf of this electrochemical cell under standard conditions. (3)
6.3 After a while the emf of this electrochemical cell decreases. Explain this observation by referring to the concentration of the electrolytes. (2) - Electrochemical cells such as motor car batteries with plastic casings can harm the environment if not disposed of safely. Suggest TWO ways how motor car batteries can be safely disposed of. (2) [14]
Solutions
1. Galvanic/voltaic cell ✓ (1)
2. Incomplete circuit/No salt bridge ✓ (1)
3. 0,76 V ✓ (1)
4. Zero ✓ (1)
5. Zn(s) → Zn2+(aq) + 2e– ✓ (1)
6.1 Mg. ✓ Mg is oxidised ✓ (2)
6.2 E0cell = E0cathode – E0anode ✓ = 0,76 – (–2,36) ✓ = 1,6 V ✓ (3)
6.3 As the cell functions, the concentration of zinc ions (reactants) decreases ✓ relative to the standard conditions and the concentration of magnesium ions (products) increases relative to standard conditions. The reverse reaction starts opposing the forward reaction causing the emf to decrease relative to standard conditions. ✓ (2)
7. Neutralise acid before disposal. ✓ Recycle plastic casing and lead electrodes ✓ (2) [14]
Activity 16
In 1780, Luigi Galvani discovered that when copper and zinc metal were connected to each other and if each free end touched different parts of the same nerve of a frog leg at the same time, the frog’s leg contracted. He called this “animal electricity”.
- Briefly explain what this “animal electricity” really was. (1)
The diagram shows an electrochemical cell setup under standard conditions using aluminium (Aℓ) and nickel (Ni) electrodes. AℓCℓ3(aq) and NiCℓ2(aq) are used as the electrolytes, and a solution of sodium nitrate (NaNO3(aq)) is used in the salt bridge.
Answer each of the following questions on this electrochemical cell:
- The diagram indicates that electrons flow from metal X to metal Y.
Identify:
2.1 Metal X (1)
2.2 Electrolyte B (1) - What is the concentration of electrolyte B? (1)
- Write down the FORMULA of the substance that moves towards metal Y in the salt bridge. (1)
- Write down the half-reaction that occurs at the cathode of this cell. (2)
- Calculate the reading on the voltmeter. (3)
- State what happens to the concentration of metal ions in the solution containing electrolyte as time goes by? (1)
- 8.1 Consider 5.7 again and recall your answer. What effect does this have on the voltmeter reading? (1)
8.2 Briefly explain your answer to 5.8.1. (2) [14]
Solutions
- The chemical reaction between the zinc and the copper r eleased electrical energy ✓ (1)
- 2.1 Metal X: Aℓ ✓ (1)
2.2 Electrolyte B: NiCℓ2 ✓ (1) - 1mol·dm–3 ✓ (1)
- NO3– ✓ (1)
- Ni2++ 2e– → Ni ✓ ✓ (2)
- E0cell = E0oxidising agent − E0reducing agent ✓ = –0,25V–(–1,66V) ✓ =1,41V✓ (3)
- Increase ✓ (1)
- 8.1 The voltmeter reading decreases ✓ (1)
8.2 As the [Aℓ3+] increases, ✓ the reverse reaction in the Aℓ Aℓ3+ half-cell:
Aℓ ⇌ Aℓ3+ +3e– is favoured.✓ (2)
OR
The tendency of the net reaction Aℓ + Ni2+ → Aℓ3+ + Ni to proceed from left to right is reduced. This lowers the electrode potential of this half-cell, resulting in a lower cell potential. [14]
Activity 17
Tina wants to investigate the effect of the area of the metal plates used as electrodes in a galvanic cell on the emf of the cell. She sets up the following Zn/Pb cell under standard conditions and measures the emf.
- Which electrode will show an increase in mass when this cell is functioning? (1)
- Write down the equation for the half-reaction occurring at the anode. (2)
- Calculate the emf that you would expect Tina to read on the voltmeter. (3)
- Name TWO variables that should be controlled for during this investigation. (2)
- Tina now replaces the two metal plates with ones of larger surface area, and takes the readings again.
5.1 How would you expect the new emf to compare with the one calculated in Question 6.3? (Only write SMALLER THAN, LARGER THAN or EQUAL TO) (1)
5.2 Explain your answer to Question 5.1 (1) - Tina now connects a resistor of low resistance across terminals A and B. She notes that the reading on the voltmeter immediately drops.
6.1 Give a reason for this observation. (1)
6.2 After some time she observes a further drop in the reading on the voltmeter. Give a reason for this observation. (2) [13]
Solutions
- Pb ✓
- Zn → Zn2+ + 2e– ✓✓
- E0cell = E0oxidising agent − E0reducing agent✓ –0,13 – (–0,76) ✓ =0,63V✓ (3)
- Temperature, ✓ (initial) concentration of electrolytes ✓(2)
- 5.1 Equal to ✓ (1)
5.2 Area/size of electrodes has no effect on the emf of a cell. It is still a standard cell ✓ (1) - 6.1 the cell has internal resistance ✓ (1)
6.2 The emf decreases as the concentration of Pb2+(aq) decreases. ✓/The cell is running flat as the electrolyte concentration in the Pb cell decreases. ✓✓ (any 2) (2) [13]
Activity 18
Electrolysis is an important industrial process used to decompose compounds, extract metals from their ores and to purify metals like gold or copper.
The simplified diagram below represents an electrolytic cell used to purify copper.
- Define the term electrolysis. (2)
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process. (2)
- Explain why the concentration of the copper(II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum. (2)
- Why is the sludge of economic importance? (1) [11]
Solutions
- The process in which electricity is used to bring about a chemical change / decompose / break compounds into components ✓✓(2)
OR
A process in which electrical energy is converted to chemical energy. - P. ✓
P is the positive electrode /anode ✓ (2)
OR
Oxidation takes place at the positive electrode/anode - Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
- Pt and Ag are both weaker reducing agents ✓ (than copper) and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent (than Pt and Ag) and will be oxidised - The rate at which copper is oxidised at the anode, ✓ is equal to the rate at which copper ions are reduced at the cathode. ✓ (2)
- Contains platinum and silver that are valuable/expensive metals ✓ (1) [11]
ACIDS AND BASES GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
- Summary
- Properties of acids and bases
- Common acids
- Common bases
- Mono- and polyprotic acids
- Conjugate acid-base pairs
- Ampholyte (amphiprotic) substance
- Salt hydrolysis
- Acid-base indicators
- Acid-base titrations (volumetric analysis)
- Preparing a standard solution
- Dilution of solutions
- Acid-base titration calculations
- The Equilibrium Constant (Kc) (The Law of Mass Action)
- Ka and Kb values
- The relationship between Ka and Kb for a substance
- Auto-ionisation of water
- Equilibrium constant for water (Kw)
- The pH scale
- pH Calculations for strong acids and bases
- Summary: Strong and weak acids and bases
- Summary: Concentrated and dilute acids and bases
Acids and bases
4.1 Summary
| Acid | (Arrhenius theory): is a substance that produces hydrogen ions (H+) / hydronium ions (H3O+) when it dissolves in water (Brønsted-Lowry theory): is a proton (H+ ion) donor. |
| Strong acid | Ionises completely in water to form a high concentration of H3O+ ions. Examples of strong acids are hydrochloric acid (HCℓ), sulphuric acid (H2SO4) and nitric acid (HNO3). |
| Weak acid | Ionises incompletely in water to form a low concentration of H3O+ ions. Examples of weak acids are ethanoic acid (CH3COOH) and oxalic acid (COOH)2. |
| Concentrated acid | Concentrated acids contain a large amount (number of moles) of acid in proportion to the volume of water. |
| Diluted acid | Dilute acids contain a small amount (number of moles) of acid in proportion to the volume of water. |
| Base | (Arrhenius theory): is a substance that produces hydroxide ions (OH–) when it dissolves in water. (Brønsted-Lowry theory): is a proton (H+ ion) acceptor. |
| Strong base | Dissociates (breaks up) completely in water to form a high concentration of OH– ions. Examples of strong bases are sodium hydroxide (NaOH) and potassium hydroxide (KOH). |
| Weak base | Dissociates/ionises incompletely in water to form a low concentration of OH– ions. Examples of weak bases are ammonia (NH3), calcium carbonate (CaCO3), potassium carbonate (K2CO3) , and sodium hydrogen carbonate (NaHCO3). |
| Concentrated base | Concentrated bases contain a large amount (number of moles) of base in proportion to the volume of water. |
| Diluted base | Dilute bases contain a small amount (number of moles) of base in proportion to the volume of water. |
| Equivalence point | is the point at which the acid / base has completely reacted with the base/acid. |
| End point | is the point where the indicator changes colour. |
| Ionisation | a process that takes place when a covalent compound reacts with water to form new ions OR Breaking up of a molecule into charged components (ions). In acid-base reactions this usually means dissolving in water. |
| Dissociation | a process that take place when an ionic compound dissolves in water allowing the ions in the compound to separate. The same as ionisation. |
| Hydrolysis | ionisation of a salt in water, or, more generally, splitting a molecule by reacting it with water (e.g. in organic chemistry). |
| Ampholyte (Amphiprotic) | is a substance that can act either as an acid or a base. example: Water (H2O) |
Activity 1
1. An Arrhenius acid is a substance that
- Accept a proton
- Donates a proton
- Produces H+ in an aqueous solution
- Produces OH– in an aqueous solution (2)
2. Which of the following is an example of the strong base
- CaCO3
- KOH
- K2CO
- NaHCO3 (2)
An aqueous solution that contains more hydronium ions than hydroxyl ions is a(an)
- Acidic solution
- Neutral solution
- Basic solution
- Standardised solution (2)
A solution that has a large amount of dissolved substances in proportion to the volume of water
- Strong solution
- Weak solution
- Concentrated solution
- Diluted solution (2) [8]
Solutions
- D✓✓
- B ✓✓
- A✓✓
- C✓✓ [8]
4.2 Properties of acids and bases
| ACIDS | BASES | |
| PROPERTIES |
|
|
| ACID-BASE THEORIES | ARRHENIUS | |
|
| |
| BRØNSTED-LOWRY | ||
|
| |
| REACTIONS WITH WATER |
|
|
NB: NOTE:
You must know the Arrhenius and the Brønsted-Lowry definitions for acids and bases, BUT we will ALWAYS use the Brønsted-Lowry theory for all further discussions of acids and bases.
Activity 2
1. Which of the following is the property of an acid
- Decreases H3O+ ion concentration in solution
- Decreases OH– ion concentration in solution
- Increases OH– ion concentration in solution
- Increases the pH of a solution (2) [2]
Solution
1. B✓✓ [2]
4.3 Common acids
| ACID | FORMULA | STRONG / WEAK | EXAMPLES & USES | ||
| Hydrochloric acid | HCℓ | Strong | IONISES ALMOST COMPLETLY IN WATER |
|
| Sulphuric acid | H2SO4 | Strong | Used
Used
| ||
| Nitric acid | HNO3 | Strong | Used
| ||
| Oxalic acid | (COOH)2 | Weak | IONISES PARTIALLY IN WATER | Used
| |
| Phosphoric acid | H3PO4 | Weak | Used
| ||
| Ethanoic acid | CH3COOH | Weak | Vinegar Used
| ||
| Carbonic acid | H2CO3 | Weak | Used
|
Hint:
- Strong acid: HCℓ; H2SO4; HNO3
- Weak acid: CH3COOH; (COOH)2
- Strong base: NaOH; KOH; Mg(OH)2
- Weak base: NH3; NaHCO3 ; CaCO3
4.4 Common bases
| BASE | FORMULA | STRONG/WEAK | EXAMPLES & USES | ||
| Sodium hydroxide (Caustic soda) | NaOH | Strong | DISSOCIATES COMPLETLY IN WATER | Used
|
| Potassium hydroxide (Caustic potash) | KOH | Strong | Used
| ||
| Magnesium hydroxide | Mg(OH)2 | Strong | Used
| ||
| Calcium hydroxide (Slaked lime) | Ca(OH)2 | Strong | Used
| ||
| Sodium carbonate (Washing soda) | Na2CO3 | Weak | DISSOCIATES / IONISES PARTIALLY IN WATER | Used
| |
| Calcium carbonate (Limestone) | CaCO3 | Weak | Found in marble and sea shells. Used
| ||
| Ammonia | NH3 | Weak | Used
| ||
| Sodium bicarbonate (Baking soda) | NaHCO3 | Weak | Used
|
4.5 Mono- and polyprotic acids
Acids can be classified according to the number of protons (H+) that they can donate.
NB: Monoprotic acids have only one proton (H+) to donate. Polyprotic acids can donate two or three protons. The protons are donated in steps as shown in the examples in the table below.
Monoprotic acids | Polyprotic acids Can donate more than one proton (H+) | |
Diprotic acids | Triprotic acids | |
| HCℓ, HNO3, CH3COOH | H2SO4, H2CO3 | H3PO4 |
E.g. | E.g. | E.g. H3PO4 → H+ + H2PO4− H2PO4− → H+ + HPO42− HPO42− → H+ + PO43− |
4.6 Conjugate acid-base pairs
4.6.1 Acid-base reactions
hint “Conjugate” is from Latin, it means literally “yoked together” or to be a couple.
Acid-base reactions take place simultaneously. The acid donates a proton to the base, while the base accepts the proton from the acid.
NB: DEFINITION
An acid-base (protolytic) reaction is a reaction in which a proton (H+) is transferred
4.6.2 Conjugate acids and bases
CONJUGATE ACIDS | CONJUGATE BASES |
When an acid donates a proton (H+), a conjugate base is produced. | When a base receives a proton (H+), a conjugate acid is produced. |
| acid ⇌ H+ + conjugate base | base + H+ ⇌ conjugate acid |
| Examples: HCℓ ⇌ H+ + Cℓ− acid conjugate base H2SO4 ⇌ H+ + HSO4− acid conjugate base | Examples: OH− + H+ ⇌ H2O base conjugate acid HSO4− + H+ ⇌ H2SO4 base conjugate acid |
Activity 3
1. In the reaction: H2SO4(aq) + H2O(ℓ) ⇌ HSO4–(aq) + H3O+(aq), the Brønsted-Lowry bases are:
- H2O and H3O+
- H2SO4 and H3O+
- HSO4– and H3O+
- H2O and HSO4– (2) [2]
Solution
1. D ✓✓ [2]
Activity 4
Find the conjugate bases and conjugate acids.
| Acid | Conjugate base | Base | Conjugate acid |
HCℓ | Cℓ− | ||
HNO3 | NO3− | ||
H2SO4 | HSO4− | ||
HSO4− | SO42− | ||
H3PO4 | H2PO4− | ||
H2PO4− | HPO42− | ||
HPO42− | PO43− | ||
H2CO3 | HCO3− | ||
HCO3− | CO32− | ||
CH3COOH | SO42− | ||
(COOH)2 | HSO4− | ||
H2O | OH− | ||
NH4+ | NH3 | ||
H3O+ | H2O |
[28]
Note that some of these are marked in BOLD. That is because they are “amphiprotic”. We will see what this means later on.
Solution
1. Check your answers
Acid | Conjugate base |
HCℓ | Cℓ− |
HNO3 | NO3− |
H2SO4 | HSO4− |
HSO4− | SO42− |
H3PO4 | H2PO4− |
H2PO4− | HPO42– |
HPO42− | PO43− |
H2CO3 | HCO3− |
HCO3− | CO32− |
CH3COOH | CH3COO− |
(COOH)2 | C2O4H− |
H2O | OH− |
NH4+ | NH3 |
H3O+ | H2O |
Base | Conjugate acid |
Cℓ − | HCℓ |
NO3− | HNO3 |
HSO4− | H2SO4 |
SO42− | HSO4− |
H2PO4− | H3PO4 |
HPO42− | H2PO4− |
PO43− | HPO42− |
HCO3− | H2CO3 |
CO32− | HCO3− |
SO42− | HSO− |
HSO4− | H2SO4 |
OH− | H2O |
NH3 | NH4+ |
H2O | H3O+ |
[28]
Steps to follow when identifying conjugates
- Find the acid on the left hand side of the arrow. Label it acid1.
- Find the conjugate base of this acid on the right hand side of the arrow. Label it base1.
- Draw a bracket to show that these two form an acid-base conjugate pair.
- Find the base on the left hand side of the arrow. Label it base2.
- Find the conjugate acid of this base on the right hand side of the arrow. Label it acid2.
- Draw a bracket to show that these two form an acid-base conjugate pair.
e.g. Worked example 1
For each of the following reactions, indicate the acid-base conjugate pairs.
- HCℓ(g) + NH3(g) ⇌ Cℓ−(aq) + NH4+(aq)
- H2SO4(g) + H2O(ℓ) ⇌ HSO4−(aq) + H3O+(aq)
- H3PO4(g) + OH−(aq) ⇌ H2PO4−(aq) + H2O(ℓ)
Solutions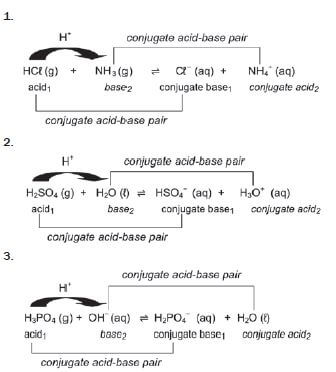
4.7 Ampholyte (amphiprotic) substance
An ampholyte:
- acts as a base in the presence of an acid and
- acts as an acid in the presence of a base.
NB: Ampholyte is a substance that can act as either a base or acid.
Example:
Water (H2O) is an ampholyte:
Water as an acid | Water as a base |
H2O(ℓ) + NH3(g) ⇌ OH−(aq) + NH4+(aq) | HCℓ(g) + H2O (g) ⇌ Cℓ−(aq) + H3O+(aq) |
Example:
Show that hydrogen sulphate ion (HSO4−) is an ampholyte.
The hydrogen sulphate ion (HSO4−) donates a proton when it acts as an acid | The hydrogen sulphate ion (HSO4−) accepts a proton when it acts as a base |
HSO4−(aq) ⇌ H+(aq) + SO42−(aq) | HSO4−(aq) + H+(aq) ⇌ H2SO4(aq) |
Activity 5
In the acid-base equilibrium formed by adding HSO4– and OH– the acids are:
- HSO4– and H2SO4
- HSO4– and H2O
- SO42– and H2SO–
- SO42– and H2O (2)
Which of the following is amphiprotic in water?
- SO2
- SO32–
- HSO3–
- H2SO3 (2) [4]
Solutions
1. B✓✓ 2. C✓✓ [4]
4.8 Salt hydrolysis
A salt is formed in the reaction between an acid and a base. When an ionic salt dissolves in water, the ions in the salt dissociate. These ions react with the water and new ions form.
NB: Hydrolysis occurs when a salt (a compound made of a metal + non-metal portion) reacts with water. Hydrolysis, more generally, is the splitting of any compound by reacting it with water. In this chapter, we only deal with the hydrolysis of salts. (Salt is not just what you have on your table; that is just one type of salt, specifically NaCℓ. Any metal combined with any non-metal is a salt).
e.g. Worked example 2
To determine whether the solutions of each of the following salts are:
- acidic or basic, and the pH of each solution is above or below 7?
- NH4NO3(aq) (2)
- K2SO4(aq) (2)
Solutions
In summary:
Remember that acids and bases react with each other to form new compounds:
We can determine whether the salt solution is basic or acidic by comparing the strengths of the reacting acids and bases.
- A salt formed between a strong acid and a weak base is an acidic salt, for example NH4Cℓ.
- When a salt reacts with water to form hydronium ions (H3O+), the solution is acidic (pH < 7).
- A salt formed between a weak acid and a strong base is a basic salt, for example NaCH3COO.
- When a salt reacts with water to form hydroxyl ions (OH−), the solution is basic (pH > 7).
- Neutral salt is formed when a strong acid and a strong base are neutralized in the reaction.
To determine the approximate pH of salts in salt hydrolysis
H2O forms H3O+ | H2O forms OH− |
|
|
Hydrolysis reaction: | Hydrolysis reaction: |
REMEMBER:
- When water reacts with a salt to form hydronium ions (H3O+), the solution is acidic (pH < 7).
- When a salt reacts with water to form hydroxyl ions (OH–), the solution is basic (pH > 7).
- The ions that don’t react are called spectator ions.
- Positive spectator ions: cations from Groups I and II e.g. K+ and Mg2+
- Negative spectator ions e.g. SO42–; Cℓ–; NO3–.
| Salt of | Nature of solution
| pH in an aqueous solution | Example | |
| Acid | Base | |||
| Strong | Strong | Neutral | pH = 7 | NaCℓ(aq) (HCℓ + NaOH) |
| Weak | Weak | Neutral | pH = 7 | CH3COONH4(aq) (CH3COOH + NH3) |
| Strong | Weak | Acidic | pH < 7 | NH4Cℓ(aq) (HCℓ + NH3) |
| Weak | Strong | Basic | pH > 7 | CH3COONa(aq) (CH3COOH + NaOH) |
Step by step
Step 1: Write down the formula of the acid and of the base that reacted to form the salt.
Step 2: Write down whether the acid is strong or weak.
Step 3: Write down whether the base is strong or weak.
Step 4: Is the salt solution acidic or basic?
e.g. Worked example 3
Determine whether each of the following salt solutions are acidic, basic or neutral.
a) Na2SO4(aq) b) NH4NO3(aq)
Solution
- Acid + Base b). Acid + Base
H2SO4 + NaOH HNO3 + NH3
Strong Strong Strong + Weak
∴ neutral solution ∴ acidic solution
4.9 Acid-base indicators
Indicators:
- can be used to determine whether a solution is acidic or basic;
- are weak acids that are in equilibrium with their conjugate bases (or vice versa) and
- have complex structures and formulae which will simply be represented as HIn (H followed by In for “indicator”).
NB: DEFINITION
- An indicator is a substance that changes colour in the presence of an acid or a base.
Indicator in equilibrium | |
| colour 1 | colour 2 |
Indicator in acid
| Indicator in base
|
e.g. Worked example 4
Bromothymol blue is an acid-base indicator. The colours it exhibits (shows) can be represented as follows:
- HIn ⇌ H+ + In−
yellow blue
A test tube contains a solution to which a drop of bromothymol blue has been added. The solution appears blue.
- What will you observe if a few drops of concentrated hydrochloric acid are added to the test tube?
- Explain your answer.
Solutions
- The colour of the solution changes from blue to yellow.
- Adding hydrochloric acid increases the concentration of the H+ ions in solution. According to Le Châtelier’s Principle the reverse reaction that opposes this change and decreases the [H+], will be favoured. Therefore the [HIn] increases and the colour changes to blue.
The most common indicators that are used in laboratories are:
INDICATOR | pH RANGE | COLOUR CHANGE |
methyl orange | 3,1 – 4,4 | red to orange to yellow |
methyl red | 4,2 – 6,2 | red to yellow |
litmus | 4,5 – 8,3 | red to blue |
bromothymol blue | 6,0 – 7,8 | yellow to blue |
phenolphthalein | 8.3 – 10 | pink-purple (magenta) in range, colourless outside range |
universal | 3-11 | red (3 and below); 3-6 orange/yellow; 7 green; 8-11 blue; 11 and above, violet. |
Choose the most suitable indicator for a particular titration. If you do not have Universal Indicator available, you should follow these steps:
Step by step – universal indicator
Step 1: Identify the strength of the acid and the base (pH range maximum and minimum)
Step 2: Draw a bracket to show the strength of the identified acid and base (shown as a line with arrowheads above)
Step 3: Note or mark the mid point (centre) of the bracket (or line as shown above)
Step 4: Use the indicator table to choose the indicator that shows the range of pH around the mid point (centre) of that bracket/line.
RESULT: You now have selected the appropriate indicator to use.
e.g. Worked example 5
Which one of the indicators given below will be the most suitable to be used in the titration of ethanoic acid against sodium hydroxide?
INDICATOR | pH COLOUR CHANGE RANGE | |
A | bromothymol blue | 6,0 – 7,8 |
B | phenolphthalein | 8,3 – 10 |
C | methyl orange | 3,1 – 4,4 |
D | methyl red | 4,2 – 6,2 |
Solution
Consult the table at Sections (3) and (4) above. We see Ethanoic Acid has a pH of about 2,4 (1M solution), and NaOH has a pH of about 14. The mid-point between 2,4 and 14 is about 5,8, so any indicator which changes from indicating acid to base around 5,8 would do. Answer: D; methyl red.
4.10 Acid-base titrations (volumetric analysis)
Titrations are used to experimentally determine the concentration of an unknown acid or base. When the titration results are used to determine the concentration of the unknown solution it is called a volumetric analysis.
Method and apparatus for an acid-base titration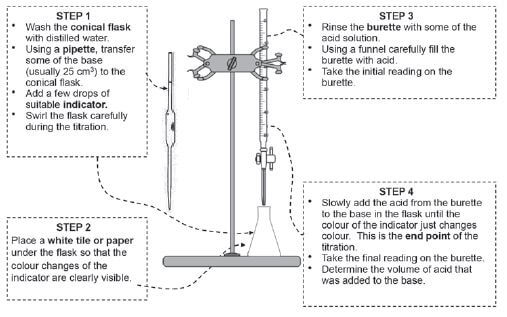
4.11 Preparing a standard solution
DEFINITION
A standard solution has a known concentration which remains constant for a period of time.
Standard solutions are often used in laboratories and it is important to know how to prepare a standard solution.
Concentration (c = n/V)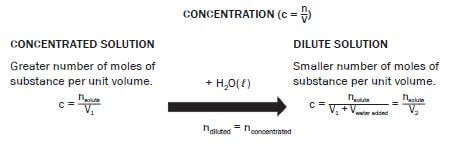
e.g. Worked example 6
hint: 1 cm3 = 1 ml ; 1 dm3 = 1 L
Aim: To prepare a 200 cm3 NaOH standard solution with a concentration of 0,5 mol∙dm–3.
Step 1: Calculate the mass of NaOH pellets required. M(NaOH) = 40 + 16 + 1 = 40 g·mol–1
Step 2: Use an electronic scale (balance) to measure off ± 4,4 g NaOH.
Note:
A specific mass of NaOH(s) can’t always be measured because it consists of pellets (not a fine powder).
Step 3: Note the exact mass of NaOH on the scale.
Step 4: Transfer the NaOH pellets to a volumetric flask. Add 100 cm3 of distilled water to the flask and seal it with a stopper. Shake the flask carefully until all the solute (NaOH) has dissolved.
Step 5: Slowly fill the flask to the calibration mark on the neck of the flask.
Step 6: Now calculate the exact concentration of the prepared solution – use the mass that was noted on the scale in Step 3. 
- n = m = 4,1 = 0,103 mol and c = n = 0,103 = 0,52 mol.dm-3
M 40 V 0,3
∴ a standard NaOH(aq) solution of concentration 0,52 mol·dm−3 has been prepared.
| Solute: | Solvent: | Solution: |
The substance (usually a solid) that is dissolved in the solvent. | The substance (usually distilled water) in which the solute is dissolved | The mixture of the solute and the solvent. |
 | ||
4.12 Dilution of solutions
We sometimes need to dilute a solution so that we can use it in a laboratory. We do this by taking a small amount of the solution and adding distilled water to it.
When diluting a solution, we need to know:
- the exact amount of distilled water that needs to added as well as
- the exact concentration of the dilute solution
In symbols: | c1 : concentration 1 (mol·dm–3) |
Remember:
- When a solution is diluted, the number of moles of substance remains constant.
- Only the volume of the solution changes (hence n is omitted in this equation.)
e.g. Worked example 7
Solution 1 has a concentration of 0,2 mol∙dm–3. Exactly 150 cm3 of solution A is transferred to a beaker 2 and 250 cm3 of distilled water is added to the beaker. Calculate the concentration of the diluted solution.
c1V1 = c2V2
(0,2)(0,15) = c2(0,4)
c2 = (0,2)(0,15)
0,4
= 0,075 mol.dm-3- The volume of the final solution (V2) is:
V2 = V1 = Vwater
=150 + 250 = 400cm3 - 400cm3
400 ÷ 1000 = 0,4 dm3 - convert to dm3
4.13 Acid-base titration calculations
In symbols:
- na = cava
nb cbvb - c = n
V
where OR c = m
MV
n = m
M- n : mol (mol)
- c : concentration (mol·dm–3)
- V : volume (dm–3)
- a : acid
- b : base
e.g. Worked example 8
During a titration 20 cm3 diluted H2SO4 precisely neutralises 25 cm3 of a NaOH solution. If the concentration of the H2SO4 solution is 0,5 mol∙dm–3, calculate the concentration of the NaOH.
Solution
First write down the balanced reaction.
1 H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + H2O(ℓ)
na = 1
nb = 2
Va = 20 cm3
Vb = 25 cm3
ca = 0,5 mol∙dm–3
cb = ?
Find the mol ratio of
- acid : base
H2SO4 : NaOH
1 : 2 - na = cava
nb cbvb - 1 = (0,5)(20)
2 cb(25) - 1.cb (25) = (0,5)(20)(2)
- cb = 0,8 mol∙dm–3
e.g. Worked example 9
TITRATION CALCULATIONS
Eight grams (8,0 g) of sodium hydroxide are dissolved in 350 cm3 of distilled water. 15 cm3 of this solution neutralises 20 cm3 of a sulphuric acid solution. The balanced equation for this reaction is:
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(ℓ)
Calculate the concentration of the sulphuric acid solution.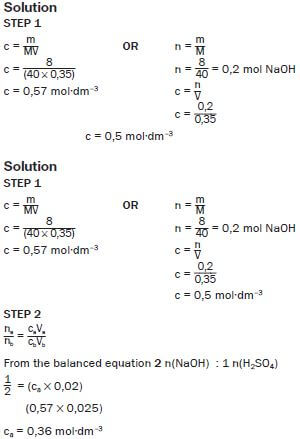
e.g. Worked example 10
The titration of oxalic acid with sodium hydroxide
Part 1:
Aim: To prepare a 250 cm3 of oxalic acid standard solution with a concentration of 0,08 mol∙dm–3
Solutions
Step 1
- Measure between 2,5 and 2,7 g of pure oxalic acid hydrate crystals (COOH)2 · 2H2O into the glass beaker and weigh again.
- Add 30-60 cm3 of distilled water to the glass beaker and dissolve the crystals.
- Transfer the solution into a clean 250 cm3 volumetric flask.
- Rinse the beaker with 15-20 cm3 of distilled water and pour this solution into the volumetric flask and repeat. This will ensure that all of the oxalic acid is transferred into the volumetric flask.
- Fill the volumetric flask to within about 2 cm of the mark and allow it to sit for a minute. This will allow any water clinging to the edges of the neck to drain into the flask. Using an eyedropper, fill the flask to the calibrated mark with water.
- Stopper (close) the flask and mix the solution by repeated inversion (turning upside-down) and swirling. This requires about 30 inversions and takes close to 1 minute.
Step 2
- Calculate the exact concentration of the oxalic acid solution using the mass of acid used and the volume of the volumetric flask and record the concentration of your solution on the bottle.
If you have used the following
- Mass oxalic acid crystals 2,6 g
- Final volume solution 250,0 cm3
Using the above information to calculate the number of moles and concentration of oxalic acid crystals.
Part 2:
Procedure to perform (to do) a titration of sodium hydroxide with oxalic acid.
Aim: use a standard solution of oxalic acid to determine the concentration of sodium hydroxide
H2C2O4(aq) + 2 NaOH(aq) → 2 H2O(ℓ) + Na2C2O4(aq)
Solutions
- Measure exact 25,00 cm3 of the oxalic acid standard solution (c = 0,084 mol·dm–3) into a flask and add few drops of phenolphthalein.
- In this titration the oxalic acid solution is acidic and therefore phenolphthalein will be colourless.
- The sodium hydroxide solution will be added drop wise (drop by drop) from a burette into the flask containing the oxalic acid and indicator.
- As the sodium hydroxide is added to the flask it will react with the oxalic acid and be neutralized.
- At the point where all of the oxalic acid is reacted, the next drop of sodium hydroxide will make the entire solution basic and it will turn pink. At this point you have completed the titration.
Results (If the following data and results were obtained after at least three trials)
- Volume oxalic acid solution 25,00 cm3
- Volume sodium hydroxide to titrate 19,43 cm3
Mole of sodium hydroxide reacted:
- na = cava
nb cbvb
First write down the balanced reaction.
- 1 H2C2O4(aq) + 2 NaOH(aq) → 2 H2O(ℓ) + Na2C2O4(aq)
Find the mol ratio of acid : base ... ratio of H2C2O4 : NaOH = 1 : 2
- na = 1
- nb = 2
- Va = 20 cm3
- Vb = 25 cm3
- ca = 0,5 mol·dm–3
- cb = ?
na = cava
nb cbvb
1 = (0,084)(25)
2 cb(25)
1· cb (19,43) = (0,084)(25)(2)
cb = 0,216 mol·dm–3
Part 3:
In order to determine the concentration of acetic acid in the vinegar solution you will titrate it with the standardized sodium hydroxide solution. The equation for this reaction is
1 CH3COOH(aq) + 1 NaOH(aq) → H2O(ℓ) + CH3COONa(aq)
The following data was obtained during titration
Data
- Volume vinegar solution 25,00 cm3
- Volume sodium hydroxide titrated 22,84 cm3
Calculations for determining concentration of acetic acid in vinegar First write down the balanced reaction.
1 CH3COOH(aq) + 1 NaOH(aq) → H2O(ℓ) + CH3COONa(aq)
Find the mol ratio of acid : base ... CH3COOH : NaOH = 1 : 1
From a balanced equation 2 n(NaOH) : 1 n(H2SO4)
In order to get the best precision possible, you should repeat each titration until you get 3 trials that are within 1% of each other.
4.14 The Equilibrium Constant (Kc) (The Law of Mass Action)
NB
Solids and pure liquids are omitted from the Kc expression as their concentration is [1], as multiplying by 1 has no effect.
The concentrations of all the compounds (solutions and gases) in a closed system in dynamic chemical equilibrium are related by a mathematical equation. The numerical value of this equation is called the equilibrium constant (Kc).
In the hypothetical equation below the equilibrium expression for this reaction is:
- 2P + 3R ⇋ 2S + 4T
Kc = [S]2 × [T]4
[P]2 × [R]3
hint
Kc: equilibrium constant (no unit)
[substance]: concentration of reactant or product (in mol·dm–3) mol: number of moles of each compound in the balanced reaction equation.
NB
- This is the correct way of writing the Kc expression
- The coefficients are the moles of each reactant and product in the balanced equation
- The product of the concentration of reactants (not to be added, but multiplied!!), raised to the power of the number of moles is the numerator
- The product of the concentration of products (not to be added, but multiplied!!), raised to the power of the number of moles is the denominator
- The concentrations used in the Law of Mass Action is the [reactant]equilibrium and [product]equilibrium (NOT initial concentrations!! i.e. the concentrations of reactants and products at equilibrium)
4.15 Ka and Kb values
Since the ionisation of a weak acid is an equilibrium, a chemical equation and an equilibrium constant expression can be written as follows. Remember that square brackets means “concentration”, or moles per dm3.
- HA + H2O ⇋ H3O+ + A– K = [H3O+][A–]
[HA]
The equilibrium constant for the ionisation of an acid is called the acid ionisation constant (Ka).
A similar expression can be written for bases:
- A + H2O ⇋ HA+ + OH- Kb = [OH–][HA+]
[A–]
The equilibrium constant for the ionisation of a base is called the base ionisation constant (Kb)
NB
An acid or base’s strength refers to its degree of ionisation. A strong acid will completely ionise in water while a weak acid will only partially ionise. Since there are different degrees of ionisation, there are different levels of weakness. Fortunately, there is a simple quantitative way of expressing this.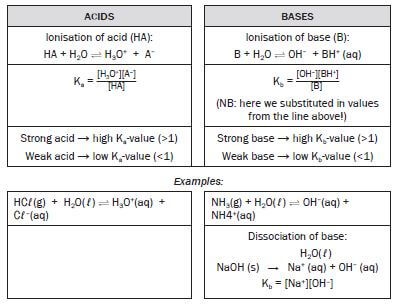
Activity 6
Do you think a strong acid will have larger or smaller Ka value? Explain your answer. (3) [3]
Solution
- The strong acid will have a larger Ka value.
- A strong acid is a better proton donor, resulting in more products.
- Since the concentration of the products is in the numerator of the Ka expression, the stronger the acid, the larger the Ka. [3]
Activity 7
Choose the strongest base in the list below by comparing their Kb values.
Base Kb
- Ammonia, NH3 1,8 × 10–5
- Hydroxylamine, HONH2 9,1 × 10–9
- Ethylamine, C2H5NH2 4,3 × 10–4 (2) [2]
Solution
- C [The larger the Kb, the stronger the base] [2]
Solids and pure liquids are NEVER written in the equations for the equilibrium constant Kc, or Ka or Kb as their concentration is [1]
4.16 The relationship between Ka and Kb for a substance
We know that the strength of a conjugate base is inversely proportional to the strength of the conjugate acid; i.e. weak acids produce strong conjugate bases, and vice versa.
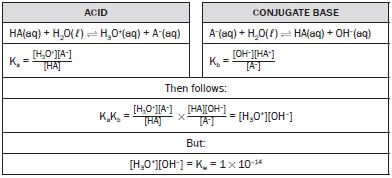
NB
You are aware that the pH scale runs from 1 to 14... now look at the values above again.
4.17 Auto-ionisation of water
Water is an ampholyte and can react as an acid (donates a H+) or a base (accepts a H+).
Two water molecules can undergo autoprotolysis or auto-ionisation. One water molecule can act as an acid and donate a H+ to the other water molecule, the base.
An acid : H2O ⇋ H+ + OH−
A base : H2O + H+ ⇋ H3O+
- Water is a weak acid and a weak base.
- Low % auto-ionisation occurs.
- Pure water is a weak electrical conductor.
Net reaction showing the conjugate acid-base pairs: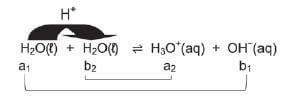
4.18 Equilibrium constant for water (Kw)
From the acid-base reaction for water the equilibrium expression for water can be written.
H2O(ℓ) + H2O(ℓ) ⇋ H3O+(aq) + OH−(aq)
- Kc = [H3O+][OH−]
- Kw = [H3O+][OH−]
Kw : equilibrium constant for water
At 25°C: Kw = 1 × 10–14
4.19 The pH scale
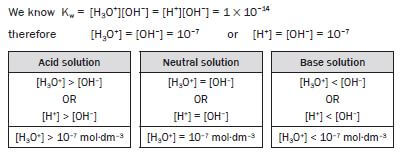
e.g. Worked example 11
Determine whether the following solutions are acidic, basic or neutral:
| A solution with |
|
| [H3O+] = 10−3 mol·dm–3 [H3O+] = 10−12 mol·dm–3 |
NB: DEFINITION
- pH of a solution is the negative logarithm of the hydronium ion concentration in a solution. pH = log [H3O+]
It is complicated to continually refer to the hydronium ion concentration. The pH scale is therefore used as a simplified method of indicating the degree of acidity of a solution.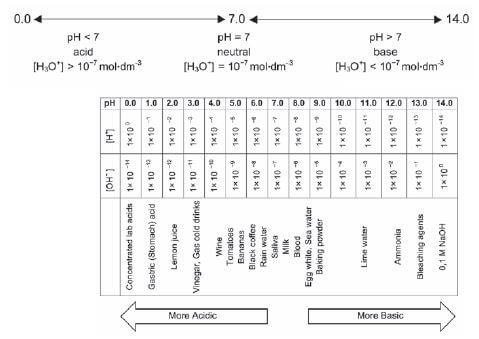
4.20 pH Calculations for strong acids and bases
To calculate the pH of a strong acid or base, these steps should be followed:
Acid | Step | Base |
0,1 mol·dm–3 of HCℓ solution | For example: | 0,5 mol·dm–3 of NaOH solution |
| Write down the ionisation reaction for the acid. HCℓ + H2O ⇋ 1 H3O+ + Cℓ− | 1 | Write down the dissociation reaction for the base. 1NaOH(s) ⇋ Na+(aq)+OH− (aq) |
Find the mol ratio of acid to hydronium ions | 2 | Find the mol ratio of base to hydroxyl ions 1 mol NaOH : 1 mol OH− |
| Determine the [H3O+] [HCℓ] = 0,1 mol·dm–3 [H3O+] = 0,1 mol·dm–3 | 3 | Determine the [OH–] |
| No step 4 for acids | 4 | Determine the [H3O+] |
Calculate the pH | 5 | Calculate the pH |
e.g. Worked example 12
Calculate the pH of a H2SO4 solution of concentration 0,6 mol∙dm–3.
Solutions
Step 1: 1 H2SO4(g) + 2 H2O(ℓ) ⇋ 2 H3O+(aq) + SO42−(aq)
Step 2: 1 mol HCℓ : 2 mol H3O+
Step 3: [H2SO4] = 0,6 mol·dm–3 ∴ [H3O+] = 2(0,6) = 1,2 mol·dm–3
Step 4: pH = − log [H3O+]
− log 1,2
0,08
4.21 Summary: Strong and weak acids and bases
Acids ionise in water to form ions:
Examples:
HCℓ(g) + H2O(ℓ) ⇋ H3O+(aq) + Cℓ−(aq)
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
| STRONG AND WEAK ACIDS | |
| STRONG ACIDS | WEAK ACIDS |
High % ionisation | Low % ionisation |
Examples: | Examples: |
| Strong acid ⇌ weak conjugate base + H+ HCℓ ⇌ Cℓ−+H+ | Weak acid ⇌ strong conjugate base + H+ CH3COOH ⇌ CH3COO− + H+ |
Remember:
- The electrical conductivity of any electrolyte is determined by the number of ions in solution. The greater the ion concentration, the higher the conductivity.
- Reaction rate is determined by the concentration of a reactant. The higher the concentration of the ions in a solution, the higher the rate of reaction.
| STRONG AND WEAK BASES | |
| STRONG BASES | WEAK BASES |
High % dissociation | Low % dissociation or ionisation |
| NaOH Sodium hydroxide KOH Potassium hydroxide | NH3 Ammonia |
| strong base + H+ ⇌ weak conjugate acid OH− + H+ ⇌ H2O | weak base + H+ ⇌ strong conjugate acid |
Remember:
- The pH of an acid is dependent on the relative concentrations of the H3O+ and OH− ions in the solution.
| In general: | |||||
pH 1 - 2 | : | strong acid | pH 12 - 14 | : | strong base |
pH 3 - 6 | : | weak acid | pH 8 - 11 | : | weak base |
| pH 7 | neutral | ||||
4.22 Summary: Concentrated and dilute acids and bases
Concentration of an acid or a base is an indication of the number of moles of solute per unit volume. Both strong and weak acids and bases can be either concentrated or diluted.
Examples: | |
1 mol·dm–3 | HCℓ(aq) : concentrated solution of a strong acid |
0,01 mol·dm–3 | HCℓ(aq) : diluted solution of a strong acid |
1 mol·dm–3 | CH3COOH(aq) : concentrated solution of a weak acid |
0,01 mol·dm–3 | CH3COOH(aq) : diluted solution of a weak acid |
1 mol·dm–3 | NaOH(aq) : concentrated solution of a strong base |
0,01 mol·dm–3 | NaOH(aq) : diluted solution of a strong base |
1 mol·dm–3 | NH3(aq) : concentrated solution of a weak base |
0,01 mol·dm–3 | NH3(aq) : diluted solution of a weak base |
Activity 8
- A standard solution is a solution ...
- at 25˚C
- of an acid or a base
- of which the volume is known
- of which the concentration is known (2)
- Consider the following ionisation equilibrium:
H2O(ℓ) ⇋ H+(aq) + OH−(aq)
The ionisation constant of water (Kw) in the above reaction increases from 1 × 10–14 at 25˚C to 9,6 × 10–14 at 60˚C. Which one of the following statements is therefore correct- [H+] > [OH−] at 60˚C
- The ionisation reaction is exothermic
- The pH increases with an increase in temperature
- The pH decreases with an increase in temperature (2)
- Consider the reaction:
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
The ionisation constant for this reaction at 25°C is 1,8 × 10–5. The equilibrium constant for this reaction at 60°C, is 3,6 × 10–7. From this information we can deduce that forward reaction ...
(Explain your choice).- is endothermic
- is exothermic
- is a redox reaction
- is a precipitation reaction (2)
- Two beakers, A and B, contain solutions of the same concentration with a pH of 2 and 4,5 respectively. Which of the following combinations is correct?
Beaker A Beaker B- Weak Acid Strong Acid
- Strong Acid Weak Acid
- Strong base Weak base
- Weak base Strong base (2)
- Andile rinses the apparatus before starting an acid-base titration experiment. Which rinsing method is likely to cause inaccurate results?
- The Erlenmeyer (conical) flask is rinsed with distilled water
- The burette is rinsed with the acids it is to be filled with
- The pipette is rinsed with the base it is to be used for
- The volumetric (measuring) flask, which is used to make up the standard solution of the base, is rinsed with distilled water. (2)
- If base X is titrated against acid Y, the pH of the solution at the end point is 8. The base X and acid Y are respectively:
X Y- NaOH CH3COOH
- Na2CO3 HCℓ
- NaOH H2SO4
- Na2CO3 CH3COOH (2) [12]
Solutions
- D ✓✓(2)
- D
 (2)
(2) - B✓✓ Kc at 25°C is greater than Kc at 60°C
Kc = [CH3COO–][ H3O+]. If the mixture is heated, the Kc decreases (2) - B✓✓ Beaker A has a low pH which indicates that it contains more H+, therefore it has ionised completely. Whilst beaker B has a high pH which indicates few H+ ions, thus it has partially ionised. (2)
- A✓✓ The Erlenmeyer (conical) flask must be rinsed with distilled water. Only the number of moles of base which was measured in the pipette must be neutralised. (2)
- A✓✓ (2) [12]
Activity 9
- Write down:
1.1 The meaning of the term diprotic acid. (2)
1.2 The formula of a diprotic acid. (1) - Magnesium hydroxide (Mg(OH)2) is often used as medicine to relieve an upset stomach. The pH of the HCℓ(aq) in a person’s stomach is 1.
2.1 Calculate the concentration of the hydrochloric acid in the person’s stomach. (3)
2.2 Will the pH in the stomach INCREASE, DECREASE or STAY THE SAME after taking in a dose of Mg(OH)2? (2)
2.3 A person takes in a dose of Mg(OH)2. Write down the balanced equation for the reaction that takes place in the stomach. (3) - A textbook states that calcium sulphate (CaSO4) is slightly soluble in water. Two learners decided to test the dam water from a local municipality for calcium sulphate. They took a 0,5 dm3 sample of the dam water and treated it with sodium carbonate solution to precipitate the calcium ions present according to the following equation:
CaSO4(aq) + Na2CO3(aq) → Na2SO4(aq) + CaCO3(s)
The precipitate is then dissolved in 30 cm3 of 0,1 mol·dm–3 HCℓ solution which converts the precipitate to aqueous calcium chloride, water and carbon dioxide according to the following equation:
CaCO3 + 2HCℓ → CaCℓ2 + CO2 + H2O
The HCℓ was in excess. They neutralised the excess HCℓ by adding 15,8 cm3 of a 0,1 mol·dm–3 NaOH solution. The equation for the reaction is:
HCℓ + NaOH → NaCℓ + H2O
Calculate the mass of calcium sulphate that was present in the sample of dam water. (10) [21]