Adele
Chemical Change - Physical Science Grade 10 Study Guide
Overview
Summary
1 Physical and chemical change
1.1 Characteristics of physical and chemical change
- A physical change is usually easy to reverse. No new chemical substance is formed; usually only a small amount of energy is involved.
- During a physical change, the mass, the number of atoms and the number of molecules remain constant. Only intermolecular forces are broken.
- An example of physical change is ice melting.
- A chemical change is usually hard to reverse. New substances are formed that have different properties from the original substances. Usually, a large amount of energy is involved in the change.
- During a chemical change, mass and the number of atoms remain constant but the number and type of molecules will change. The atoms break apart and rearrange to form new compounds.
- An example of chemical change is iron sulphide being formed from the heating together of iron and sulphur.
- When only a physical change is involved, separation methods such as filtration, distillation and paper chromatography can be used to separate a mixture. Once a chemical change has occurred, these methods cannot be used.
1.1.1 Law of conservation of matter
- The total mass of any isolated system is constant and is independent of any chemical and physical changes taking place within the system.
1.1.2 Law of constant composition
- A particular chemical compound always contains the same elements combined in the same fixed proportions by mass.
- You can work out the ratio in which the elements combine by looking at their atomic masses.
1.2 Representing chemical change
- Chemical change can be represented or shown by balanced chemical equations. A balanced chemical equation is one in which the number and type of atoms present in the reactants (the substances on the left of the arrow) equal the number and type of atoms present in the products (the substances on the right of the arrow).
- State symbols like (s), (aq), (l) and (g) are put in brackets after each substance.
- To balance a chemical equation the formulae of the substances must not be changed. This means the small numbers in a formula, for example, the 2 in H2O, may not be changed. Only the number that represents the number of molecules or formula units may change. This is the number in front of the formula.
1.3 Energy transfer in chemical change
- Just as mass and the number of atoms are conserved during a chemical change, so too is the amount of energy of the system.
- During a chemical reaction, energy is needed to break old bonds between atoms, and energy is released when new bonds form between atoms.
- If the energy released is greater than the amount needed to break the old bonds, the extra energy is given out as heat. The container in which the reaction occurs gets hot. This type of reaction is called an exothermic reaction.
- If the energy required to break the bonds is greater than the energy released when the new bonds form, energy must be put into the system. The container in which the reaction happens gets cold. This type of reaction is called an endothermic reaction.
2 Reactions in aqueous solution
2.1 Ions in aqueous solution
- Ions are elements (or groups of elements) that have lost or gained electrons.
- Negative ions are called anions and positive ions are called cations.
- An aqueous solution is a solution in which the solvent (the liquid that dissolves the solid (the solute)) is water.
- Ions in general dissolve easily in water. This process is called dissolution.
- When an ionic solid (a solid made from the bonding of positive and negative ions) is placed in water, dissolution (or the dissolving process) happens in two steps:
the ionic solid breaks up into positive and negative ions
the ions become hydrated. - Dissolution can be shown by means of state symbols. Example: NaCl(s) → Na+(aq) + Cl–(aq)
- In a water molecule, the hydrogen atoms are slightly positively charged, and the oxygen ions are slightly negatively charged. This is called a polar molecule.
- When the ions come into contact with the water, water molecules surround the negative ions. There is an attraction between the negative ion and the hydrogen atoms.
- Positive ions become surrounded because of the attraction between the positive ion and the negative oxygen of the water molecule.
- As a result, the ions cannot come together again, and so they remain in solution.
2.2 Electrolytes and ionisation
- An electrolyte is a solution that can conduct electricity. Ionic solutions are electrolytes.
- To measure the conductivity of an ionic solution, carbon electrodes are used that are attached to either end of a battery. An electrode is defined as a solid object through which electricity enters or leaves a substance.
- Cations flow towards the negative electrode and anions flow towards the positive electrode.
- The more dissolved ions there are in a solution, the greater the current that will flow in it.
- Non-ionic solutions such as sucrose (even though they can dissolve in water) cannot act as an electrolyte and conduct electricity. Solid ionic compounds also cannot conduct electricity. They have to be molten (melted) or in solution.
2.3 Precipitation reactions
- While many ionic compounds are soluble in water, some are not. There are some general rules we can use to predict whether a particular ionic compound will dissolve or not.
Compound Solubility | |
All nitrates | All are soluble. |
Salts containing potassium, sodium or ammonium | All are soluble. |
Chlorides | All are soluble, except silver, lead and mercury chloride. |
Sulphates | All are soluble, except lead sulphate, barium sul- phate and calcium sulphate. |
Carbonates | All are insoluble, except those with potassium sodium or ammonium. |
Silver bromide and silver iodide are insoluble. | |
- If two different ionic solutions are mixed, an ion from the one compound can change places with an ion from the other compound. A chemical reaction occurs. If the water from the solution is removed, two new substances will be present.
Example: BaNO3(aq) + K2SO4(aq) → BaSO4(s) + KNO3(aq)
The NO3– ion has changed places with the SO4– ion. A reaction like this is called an ion-exchange reaction. - If one of the new substances that forms is insoluble, it will form a solid and sink to the bottom of the solution. The solid that forms in such a reaction is called a precipitate.
- Chlorides, bromides and iodides (halides) will all form a precipitate if they are mixed with soluble silver nitrate. Chlorides form a white precipitate. Bromides and iodides both form creamy yellow precipitates, but the precipitate of bromides dissolves in ammonium hydroxide solution.
- Sulphates will form a precipitate if mixed with soluble barium chloride.
Example: MgSO4(aq) + BaCl2(aq) MgCl2(aq) + BaSO4(s) - Carbonates will also form a precipitate with barium chloride. In the case of carbonates, the precipitate that forms will dissolve, producing bubbles, if some nitric acid is added to it.
2.2 Other chemical reaction types
- Precipitation reactions – the driving force is the formation of an insoluble salt. Example: NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
As you can see from the state symbols, the insoluble salt silver chloride forms during the reaction. - Gas-forming reactions – the driving force is the formation of a gas. Example: Mg(s) + 2 H2O(l) → Mg(OH)2(aq) + H2(g)
The water splits into H+ and OH– ions and the OH– ions join with the magnesium
so that hydrogen is released to form hydrogen gas. - Acid-base reactions – the driving force is the transfer of protons (H+ ions). Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
The H+ ion in the acid reacts with the OH– ion in the base, causing the formation of water. Generally, the product of this reaction is some ionic salt and water. - Redox reactions – the driving force is the transfer of electrons. (You will learn much more about these reactions in a later grade.)
Example: 2Mg(s) + O2(g) → 2MgO(s)
3 Quantitative aspects of chemical change
3.1 Atomic mass and the mole concept
- In chemistry, a ‘mole’ is the unit used to measure the quantity (the number of particles) of a substance.
- One mole of any substance = 6,02 × 1023 particles. This number is known as ‘Avogadro’s number.’
- A mole is also defined as being that quantity of a substance that contains exactly the same number of particles as there are carbon atoms in 12 g of carbon. Therefore, one mole of any element has a mass (molar mass) equal to its relative atomic mass in grams.
- The relative formula mass (of an ionic substance) or the relative molecular mass of a molecule (a covalent substance) is the sum of the atomic masses of all the atoms in its formula. Therefore, the molar mass of a molecular substance is equal to its molecular mass and the molar mass of an ionic substance is equal to its formula mass.
3.2 Molecular and formula masses
3.2.1 Relationship between moles, mass and molar mass
The number of moles = total mass of substances
relative atomic mass of one atom of the substance
OR
n = m / M where n = number of moles; m = the mass of a substance in grams and M is the atomic/formula mass.
3.2.2 Water of crystallisation
- ‘Water of crystallisation’ refers to water molecules that are trapped in some crystals as their crystal lattice forms. Although they are not bonded to the crystal, they add to its mass. Therefore, the water of crystallisation must be added in when the formula mass is calculated.
Example:
Formula mass of CuSO4.5H2O is 4 × (65,5 + 32 + 16) + 5 × (1 × 2 + 16) = 252
3.3 Determining the composition of substances
3.3.1 Percentage composition
- The percentage composition of a compound is the percentage of the total mass of the substance that is made up of each element.
- To calculate the percentage mass of an element in a compound, use the following formula:
% mass = atomic mass + number of atoms of that element × 100
formula mass of the whole compound
[Example: to find the % sodium in Na2CO3
% sodium = 23 × 2 × 100 = 46 × 100 = 43,4%
(23 × 2) + 12 + (16 × 3) 106
3.3.2 Empirical formula
- The empirical formula of a compound is the simplest ratio of all the elements present in that compound. Sometimes the empirical formula and the molecular formula are the same (for example, in H2O), but if the molecular formula can be divided by one common factor (for example, in C4H8), the molecular and empirical formulas are different.
- To find the molecular formula from the empirical formula and the molecular mass:
- Calculate the relative formula mass of the empirical formula.
- Divide this into the molecular mass.
- Take that answer and multiply each subscript in the empirical formula by that number.
Example: The empirical formula is HO and the molecular mass is 34. The relative molecular mass of the empirical formula is (1 + 16). 34 ÷ 17 = 2.
Therefore HO × 2 = H2O2, which is the molecular formula.
3.3.3 Molar volume
- Molar volume of gases refers to the fact that one mole of any gas at the same temperature and pressure has the same volume as one mole of any other gas at that temperature and pressure.
- It has been found that one mole of any gas at standard temperature (which is 273 K or 0 °C) and standard pressure (which is 1,013 × 105 Pa) has a volume of 22,4 dm3. Therefore, the volume of any gas (at STP) = n × 22,4 dm3, where n = the number of moles.
3.3.4 Concentration
- The concentration of a solution is usually given in ‘moles per dm3’ or mol.dm–3.
- Concentration c = n / v where:
c = concentration in mol.dm–3; n = number of moles and v = volume in dm–3. (Note that 1000 cm3 = 1 dm3 = 1000 ml or 1 litre.)
3.4 Basic stoichiometric calculations
- Using balanced chemical equations and what you have learnt about moles, it is possible to find out how much of a certain product can be made from a certain amount of reactant.
- A balanced reaction gives you the ratio (in moles) in which substances react.
- Example: 2 H2 + O2 → 2 H2O tells us that 2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water.
- If you are given the mass of reactants and have to find the mass of the products, use the formula (n = mass / M) to convert mass of reactant to moles of reactant.
- Use the ratio given by the reaction to find the number of moles of product formed.
- Convert the number of moles of product to mass of product, again using (n = mass / M).
- If you are given dm3 of a gas at STP, you must first convert this to the number of moles, using the formula volume in dm3 = n × 22,4. (One mole of any gas at STP has a volume of 22,4 dm3.)
- If you are given the concentration of a reactant, convert this to moles using the formula n = c × V.

Magnetism and Electricity Questions and Answers Grade 10
Questions
Question 1: Multiple choice
Choose the correct answer. Write only the letter of the answer you select.
1.1 Which of the following statements about magnetic field lines is/are true?
- They surround a magnet in two dimensions.
- They cannot cross.
- They represent the direction in which the south pole of a compass will point.
- 1 and 2 only
- 2 and 3 only
- 2 only
- 1, 2 and 3 (3)
1.2 Which one of the substances below will be attracted by a magnet?
- copper
- water
- lead
- cobalt (3)
1.3 A small, neutral, metal sphere becomes charged when it is brought into contact with a positively charged rod. In the process the sphere …
- loses electrons.
- loses protons.
- gains protons.
- gains electrons. (3)
1.4 An insulated metal sphere carries a charge of –7 μC. An identical sphere carries a charge of –9 μC. The spheres are brought together to touch and are then sepa- rated. What is the charge on each sphere now?
- –16 μC
- –8 μC
- –1 μC
- +8 μC (3)
1.5 Two identical metal spheres, X and Y, are mounted on insulating stands. Sphere X has a charge of +8 μC and sphere Y is neutral. The spheres are allowed to touch and are then separated. What is the charge on X?
- 0
- –8 μC
- +4 μC
- +8 μC (3)
1.6 An insulator can be attracted by a charged plastic ruler because …
- the insulator atoms are always polarised.
- the insulator atoms become polarised when close to the charged ruler.
- an insulator contains free electrons.
- electrons in the insulator move to one side of the insulator, thereby polarising the whole insulator. (3)
1.7 The SI unit for current is …
- ampere.
- coulomb.
- ohm.
- volt. (3)
1.8 You wish to connect a circuit to measure the current in a resistor and the potential difference across it. Which circuit below is connected correctly?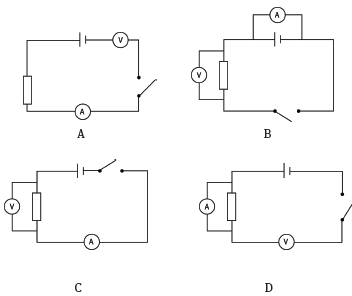 (3)
(3)
1.9 A volt can be described as a …
- coulomb per second.
- joule per ampere.
- ampere per second.
- joule per coulomb. (3)
1.10 A conductor carries a current of 2 A. What is the total charge that passes through the conductor in 4 minutes?
- 480 C
- 8 C
- 2 C
- 0,5 C (3)
1.11 Three resistors each have a resistance of 6 Ω. Which of the following correctly gives their equivalent resistance when first connected in series, then in parallel?
- 18 Ω and 3 Ω
- 18 Ω and 2 Ω
- 2 Ω and 18 Ω
- 18 Ω and 0,5 Ω (3)
1.12 Which pair of words, in order, correctly completes the following statements?
When the length of a resistor is increased, its resistance … When the thickness of a resistor is decreased, its resistance …
- increases, increases
- increases, decreases
- decreases, decreases
- decreases, increases (3)
1.13 Three resistors, each of resistance 4 Ω, are to be used to make a 6 Ω combination. Which arrangement will achieve this?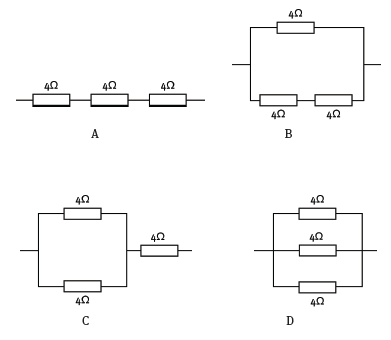 (3)
(3)
1.14 You are given this section of an electric circuit Which of the following statements is/are correct?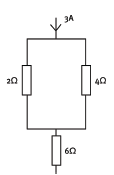
- The current in the 2 Ω resistor is 2 A.
- The current in the 4 Ω resistor is 1 A.
- The current in the 6 Ω resistor is 3 A.
- 3 only
- 2 and 3 only
- 1 and 2 only
- 1, 2 and 3 (3)
1.15 In the given circuit, the equivalent resistance between X and Y is …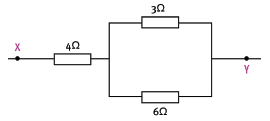
- 4,5 Ω
- 5 Ω
- 13 Ω
- 6 Ω (3)
1.16 The bulbs in this circuit are all identical. If the reading on ammeter A1 is 6 A, what is the reading on ammeter A2?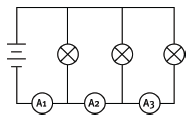
- 2 A
- 3 A
- 4 A
- 6 A (3)
1.17 The resistance of a conductor does not depend on …
- length.
- potential difference.
- temperature.
- type of material. (3)
1.18 One of the bulbs in this circuit breaks and that causes all the other bulbs to go out as well. Which bulb broke?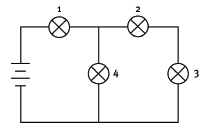
- 1
- 2
- 3
- 4 (3) 18 × 3 = [54]
Question 2: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
2.1 When a bar magnet is broken in half, one half is a north pole only and the other half is a south pole. (2)
2.2 The SI unit for resistance is the volt per coulomb, given the name ohm. (2)
2.3 In any series electrical circuit we will find the biggest potential difference across the resistor with the biggest resistance. (2)
2.4 The voltage across the terminals of a battery always decreases when the battery starts to deliver current to the circuit, due to the resistance of the battery. (2)
2.5 The total potential difference across three resistors connected in parallel is equal to the sum of the potential differences across each resistor. (2) 5 × 2 = [10]
Question 3: One-word answers
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
3.1 A region where a magnetic substance will experience a force.
3.2 A substance that will not allow a flow of charge through it.
3.3 The voltage measured across the terminals of a battery when it is not providing current to a circuit.
3.4 The SI unit for charge.
3.5 The rate of flow of charge. 5 × 1 = [5]
Question 4: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
4.1 axis on which Earth spins | A plastic |
4.2 charging by rubbing | B voltage dividers |
4.3 polarised molecules | C geographic N and S poles |
4.4 resistors in parallel | D ammeter |
4.5 measuring instrument that is con- nected in series | E induction |
F water | |
G current dividers | |
H magnetic N and S poles | |
I voltmeter | |
J tribo-electric |
5 × 2 = [10]
Question 5: Long questions
You are given a painted metal rod and a magnet. Explain how you could show whether the painted metal rod is …
5.1 non-magnetic (for instance, copper). (2)
5.2 magnetic, but not a magnet. (2)
5.3 a magnet. (2) 3 × 2 = [6]
Question 6: Long questions
6.1 Explain the difference between a magnetic field and a magnetic field line. (4)
6.2 What is meant by the ‘north pole’ of a magnet? (2)
6.3 You are given two identical magnets of equal strength. You place them with the north pole of one facing the south pole of the other, as shown.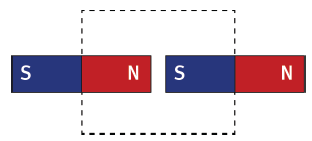
6.3.1 Draw the magnets in the area of the dotted line box, then sketch the mag- netic field pattern in the area of the box. (4)
6.3.2 How would the pattern change if both magnets were much weaker? (2) [12]
Question 7: Long questions
7.1 Explain the difference between the geographic north pole and the magnetic north pole of the Earth. (4)
7.2 What is the angle between the lines drawn between the geographic N and S, and the magnetic N and S? (1) [5]
Question 8: Long questions
8.1 Name the three particles that make up an atom, and state the charge on each. (3)
8.2 Why are the names ‘positive’ and ‘negative’ used for the two types of charge? (3) [6]
Question 9: Long questions
Plastic carrier bags are made of polythene. A strip is cut from a carrier bag and rubbed with a cloth or by pulling it through your fingers. When it is then hung from a string as shown, the ends push apart. Explain why this happens. (4)
[4]
Question 10: Long questions
10.1 Explain why a water molecule is said to be ‘polarised’. Draw a labelled sketch of a water molecule to illustrate your answer. (4)
10.2 A plastic rod is charged positively by rubbing it with cloth. When it is brought near to a thin stream of water from a tap, the water is attracted to the rod.
10.2.1 Explain in terms of transfer of charge how the rod became positively charged. (2)
10.2.2 What name if given to the process of charging an object by rubbing? (1)
10.2.3 The charge on one electron is 1,6 × 10–19 C. If 3,6 × 106 electrons were trans- ferred in the rubbing process, calculate the charge on the rod. (4)
10.2.4 Explain why the water is attracted. (4) [15]
Question 11: Long questions
11.1 How would you measure the emf of a battery? Explain why this works. (2)
11.2 Why is the emf of a battery always slightly higher than the potential difference that it can provide to an electric circuit? (4) [6]
Question 12: Long questions
Study the circuit diagram and answer the questions below.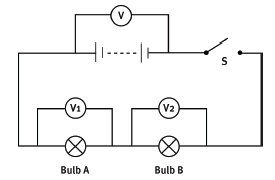
12.1 Is the resistance of a voltmeter very high or very low? Explain how this affects the measurement of voltage. (4)
12.2 When switch S is open, as shown, the reading on voltmeter V is 10 V. Switch S is then closed and the voltmeter reading drops to 9 V. Explain why. (5)
12.3 How much energy does the battery give to each coulomb of charge that it pushes out into the circuit? (1)
12.4 If the reading on voltmeter V1 is 4 volts, what is the reading on V2? (2)
12.5 How much energy is lost by each coulomb of charge as it passes through bulb A? (2)
12.6 Into what two forms of energy is it transformed in bulb A? (2) [16]
Question 13: Long questions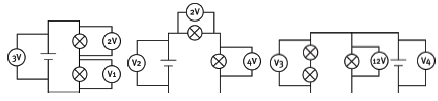
In the above circuits, the bulbs and cells are not all identical. The readings on some of the voltmeters are given. What are the readings on the other voltmeters V1 to V4? [8]
Question 14: Long questions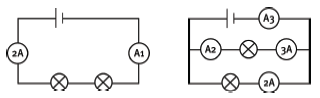
In the above circuits, the light bulbs are not all identical. The readings on some of the ammeters are given. What are the readings on the other ammeters A1 to A5? [10]
Question 15: Long questions
15.1 Define current. (2)
15.2 Name the instrument used to measure current. (1)
15.3 Does this instrument have a very high or a very low resistance? Give the reason for your answer. (3)
15.4 An electric light bulb carries a current of 0,25 A. Calculate the time taken for 30 C of charge to pass through the bulb. (4) [10]
Question 16: Long questions
16.1 State the four factors that determine the resistance of a resistor. (4)
16.2 Why are metals good conductors of electricity? (2)
16.3 Explain why a metal can get hot when carrying an electric current. (4) [10]
Question 17: Long questions
17.1 What name is given to a ‘volt per ampere’? (1)
17.2 When an electric heater is connected to the electricity mains of 240 volts, the cur- rent is 5 amperes.
17.2.1 How many volts are required to produce a current of one ampere in the bulb? (3)
17.2.2 What is the resistance of the bulb? (1) [5]
Question 18: Long questions
An electric circuit consists of a battery of four cells connected to two resistors and an ammeter all connected in series. The resistances are 6 Ω and R (representing an unknown resistance). The ammeter reads 1 A. The potential difference across the 6 Ω resistor is 6 V. A voltmeter connected across the battery reads 8 V.
18.1 Draw the circuit diagram, entering all the given information. (4)
18.2 What is the potential difference across R? Explain your answer. (3)
18.3 Is the resistance of R greater than or less than 4 Ω ? Explain your answer. (3)
18.4 What is the current in R? Give a reason for your answer. (2)
18.5 How much charge passes through the 6 Ω resistor in 1½ minutes? (4) [16]
Question 19: Long questions
Consider the circuit given in the diagram below.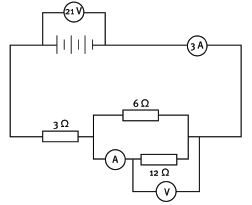
19.1 Calculate the equivalent resistance of the parallel combination. (4)
19.2 What is the total resistance of the whole circuit? (2)
19.3 If the potential difference across the 3 Ω resistor is 9 V, what is the reading on voltmeter V? (2)
19.4 If the current in the 6 Ω resistor is 2 A, what is the reading on ammeter A? (2)
19.5 How much charge passes through the 3 Ω resistor in 20 s? (4) [14]
Answers
1.1 C
1.2 D
1.3 A
1.4 B
1.5 C
1.6 B
1.7 A
1.8 C
1.9 D
1.10 A
1.11 B
1.12 A
1.13 C
1.14 D
1.15 D
1.16 C
1.17 B
1.18 A 18 × 3 = [54]
2.1 False. When a bar magnet is broken in half, each half remains being a magnet, each with a north pole and a south pole.
2.2 False. The SI unit for resistance is the volt per ampere, given the name ohm.
2.3 True.
2.4 True.
2.5 False. The total potential difference across three resistors connected in series is equal to the sum of the potential differences across each resistor. 5 × 2 = [10]
3.1 magnetic field
3.2 insulator
3.3 emf
3.4 coulomb
3.5 current 5 × 1 = [5]
Question 4: Matching pairs
4.1 C
4.2 J
4.3 F
4.4 G
4.5 D 5 × 2 = [10]
Question 5: Long questions
5.1 Hold the magnet close to the painted metal rod. If there is no attraction, the painted rod is not magnetic.
5.2 If the painted rod is attracted by both poles of the magnet, the painted rod is magnetic, but not a magnet.
5.3 If the one end of the painted rod is attracted by the north pole of the magnet, while the other end of the rod is repelled by the north pole, then the painted rod is a magnet. 3 × 2 = [6]
Question 6: Long questions
6.1 A magnetic field is simply a region where a magnet or magnetic material will experience a force. Magnetic field lines are imaginary lines that are drawn to show the direction in which the north pole of a compass would point if placed at any point on the lines.
6.2 If the magnet is suspended so it can swing freely, the north pole of the magnet is the end that will point towards to north pole of the Earth under the action of the magnetic field of the Earth.
6.3.1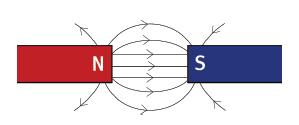
Shape of field
Direction of arrows
6.3.2 All field lines would be further apart. [12]
Question 7: Long questions
7.1 The geographic north pole is the northern point of the axis around which the Earth spins.
The magnetic north pole is the point towards which the north pole of a compass will point.
7.2 11,5° [5]
Question 8: Long questions
8.1
- proton – positive
- electron – negative
- neutron – neutral
8.2 The charge on a proton equals that on an electron, but when a proton and an electron are brought together, the charge cancels to give a total charge of zero. This is the same as considering a proton to have a charge of +1 and an electron –1, so if added together equals zero. [6]
Question 9: Long questions
When the polythene strip is rubbed, electrons are transferred onto the strip making the whole strip negatively charged. When hung over the string, the two ends of the strip repel each other, as negative repels negative. [4]
Question 10: Long questions
10.1 The oxygen atom attracts electrons in the molecule more strongly than the hydrogen atoms. This makes the oxygen end of the molecule slightly negatively charged and the hydrogen end slightly positively charged. 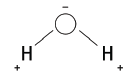
10.2.1 Negatively charged electrons were rubbed of the rod onto the cloth. This left more protons than electrons on the rod, making the whole rod positively charged.
10.2.2 tribo-electric charging
10.2.3
- Q = nqe
= (3,6 × 106) × (1,6 × 10–19)
= 5,76 × 10–13 C
10.2.4
- The positive rod attracts oxygen atoms in the water molecules and repels the hydrogen atoms. This causes the molecules to rotate, so that the whole stream is polarised with the side of the stream closest to the rod being negative and the side furthest from the rod positive. Because all oxygen atoms are now closer to the rod than hydrogen atoms, the force of attraction is stronger than the force of repulsion, so the whole stream is attracted. [15]
Question 11: Long questions
11.1 Connect a voltmeter directly to the battery. Because the voltmeter has very high resistance, the current is effectively zero. The reading on the voltmeter is the emf.
11.2 The resistance of the circuit is much lower than that of the voltmeter, so the cur- rent in the battery is now large. The battery has resistance, so some of the energy provided to the charge by the battery is used up in the battery itself, leaving less available for the circuit. [6]
Question 12: Long questions
12.1 Very high. A voltmeter is connected in parallel across the resistor(s) where it measures potential difference. By having a very high resistance, very little cur- rent passes through the voltmeter.So the voltmeter does not affect the current in the circuit.
12.2 When the switch is open, current from the battery passes only through the voltmeter of very high resistance. This current is so small that it is effectively zero. So the emf is 10 V. When the switch is closed, the current through the battery is now the same as the current in the circuit and is much larger. Be- cause the battery has resistance, some of the energy supplied by the battery is transformed into heat inside the battery, so only 9 V is available for the external circuit.
12.3 9 J
12.4 5 V
12.5 4 J
12.6 heat and light [16]
Question 13: Long questions
V1 = 1 V
V2 = 6 V
V3 = 12 V
V4 = 12 V [8]
Question 14: Long questions
A1 = 2 A
A2 = 3 A
A3 = 5 A
A4 = 2 A
A4 = 1,5 A [10]
Question 15: Long questions
15.1 Current is the rate of flow of charge.
15.2 Ammeter
15.3 Very low resistance. An ammeter is connected in series in a circuit. It must have a very low resistance so that it does not affect the current in the circuit.
15.4 I = Q / Δt Δt = Q / I = 30 / 0,25 = 120 s [10]
Question 16: Long questions
16.1 type of material; length; thickness; temperature
16.2 Some of the electrons in a metal are held loosely by the nucleus, so they are free to move.
16.3 The battery sets up an electric field in the circuit. This forces all loose electrons in the circuit to move from the negative towards the positive terminal. The electrons collide with the atoms of the metal, causing them to vibrate faster and heat up. [10]
Question 17: Long questions
17.1 ohm
17.2.1 If a voltage of 240 volts produces a current of 5 amperes, 1 ampere will be produced by (240 ÷ 5) = 48 volts
17.2.2 48 Ω [5]
Question 18: Long questions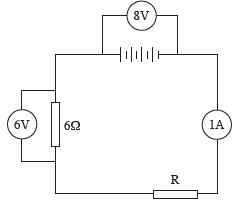
18.1 The 6 Ω resistor, resistor R and the ammeter can be connected in any order.
18.2 2 V The resistors in series are voltage dividers – they divide the voltage of the battery in proportion to the resistances of the resistors. The voltages across the two resistors together must add up to 8 V.
18.3 Less than 4 Ω. Voltage is divided in proportion to the resistance. R has the smaller proportion of the voltage, so must have the smaller resistance.
We can actually predict that the resistance of R is 2 Ω, because the ohm is defined as a volt per ampere. The current in R is 1 ampere and the potential difference across it is 2 volts, therefore 2 V A–1 = 2 Ω. We can calculate the resistance another way. The voltage of the battery is divided in proportion to the resistance. R takes ¼ of the voltage of the battery, so must have ¼ of the total resistance. If the 6 Ω resistor is ¾ of the total resistance, the other ¼ must be 2 Ω.
18.4 1 A. Current is the same at all points in a series circuit.
18.5 Time must be expressed in seconds. 1½ minutes = 90 s.
Q = I Δt = 1 × 90
= 90 C [16]
Question 19: Long questions
19.1
- Rp = 1 + 1 = 1 + 1 OR Rp = R1R2
R1 R2 6 12 R1 + R2
= 2 + 1 = 3 = 6 × 12
12 12 6 + 12
RP = 12 = 4 Ω = 4 Ω
3
19.2
- Rs = R1 + R2
= 3 + 4
= 7 Ω
19.3 9 V (Must add up to the potential difference provided by the battery.)
19.4 1 A (Branched currents must add up to mainstream current.)
19.5
- Q = I Δt
= 3 × 20
= 60 C
Magnetism and Electricity - Physical Science Grade 10 Study Guide
Overview
Summary
1 Magnetism
1.1 Magnetism
- A magnetic field is a region in space in which a magnet or ferromagnetic substance (iron, nickel or cobalt) will experience a force.
- The poles of a magnet are at the two ends of the magnet. The magnetic force is strongest at the poles.
- If a magnet is cut in half, each half will be a magnet with a north pole at one end and a south pole at the other.
- If a magnet is suspended at its centre so that it can turn freely, it will be affected by the magnetic field of the earth and settle in a north-south direction. The pole of a magnet that points towards the Earth’s north pole is called the north-seeking pole (or simply the north pole) of the magnet.
- Like poles of two magnets repel, unlike poles attract. So north repels north, but north attracts south.
- A compass is a magnet that is free to turn at its centre.
- A magnet is surrounded by a magnetic field – a region in which magnetic materials such as iron will experience a force.
- A compass is used to show the direction of a magnetic field.
- A magnetic field line shows the shape of the field and the direction that the north pole of a compass will point when placed in the field.
- Magnetic field lines point from north to south of a magnet.
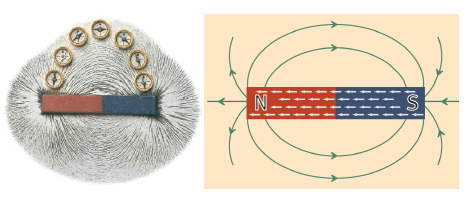
- The more closely spaced the field lines are at a point, the stronger is the field at that point, that is, the stronger the force will be on a magnetic object.
- Field lines never cross. They surround a magnet in three dimensions. For simplicity, we draw field lines in two dimensions only.
- The geographical north and south poles are the points around which the Earth rotates.
- The magnetic field of the Earth is similar to that of a bar magnet, with poles at the magnetic north and magnetic south of the Earth.
- The imaginary bar magnet inside the Earth must have its north pole at the south geomagnetic pole.
- The angle between the geographic north pole and the geomagnetic north pole is 11,5°.

- The magnetic field of the Earth protects the Earth from harmful ions of hydrogen and helium, as well as electrons emitted by the sun (solar wind). These are deflected to the poles by the magnetosphere, where they collide with the atmosphere and produce an aurora.
2 Electrostatics
2.1 Two kinds of charge
- The property of particles in atoms that enables them to attract and repel is called charge. There are only two types of charge.
- Like charges repel, unlike charges attract.
- Charges are called positive and negative because when the two types come together they cancel out to produce zero charge.
- Atoms are made up of a central nucleus comprised of positively charged protons and neutral neutrons. The nucleus is surrounded by a number of negatively charged electrons that are much smaller than protons.
- Objects become charged when electrons are either removed from them or added to them. This can be done by rubbing two materials together, called tribo-electric charging.
- An object that has an equal number of positive and negative charges is neutral.
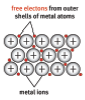
- Conductors allow a flow of charge through them. Conductors contain charges that are free to move.
- Insulators do not allow a flow of charge through them. They do not contain charged particles that are free to move.
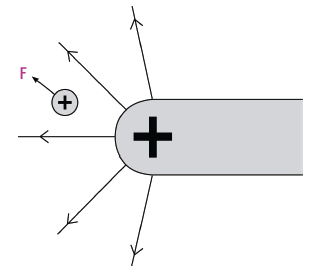
An electric field is a region in space in which an electric charge will experience a force. All charged objects are surrounded by electric fields.
- An electric field line is a line drawn with an arrow to show the direction in which a positive charge will experience a force if placed in the field.
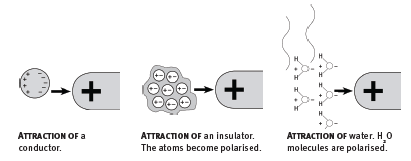
- An uncharged conductor can be attracted by a charged object. In the process, the conductor becomes polarised. Free electrons in the conductor move under the action of the electric field, making one side of the conductor negative and the other positive.
- An uncharged insulator (for instance, dust or paper) can be attracted by a charged object. Here each atom becomes polarised. Electrons in each atom move under the action of the electric field, making one side of the atom negative and the other positive.
- Water is made up of polarised molecules. The oxygen end is slightly negatively charged, and the hydrogen end slightly positively charged. A thin stream of water is attracted by a charge rod. The molecules rotate under the action of the electric field so that one side of the stream is negative and the other positive.
2.2 Conservation and quantisation
- Charge is measured in coulombs, where one coulomb is the charge carried by 6,25 × 1018 electrons.
- The principle of conservation of charge states that the net charge of an isolated system remains constant during any physical process.
- When two identical conductors that are charged are brought into contact, electrons will flow from the more negative object to the other until both have the same charge. To calculate the charge on each, simply add together the original charges and divide by two.
Q = (Q1 + Q2) / 2 - The charge on one electron (qe) is called the elementary charge.
qe = 1,6 × 10–19 C - The principle of quantisation of charge states that every charge in the universe must be a multiple of the charge on one electron.
Q = nqe where qe = 1,6 × 10–19 C.
2.2.1 Worked examples
- Two identical metal spheres A and B on insulated stands are charged. The charge on A is +6,4 μC. The charge on B is –24,6 μC. The two spheres are brought into contact, then separated.
1.1 Express each charge in C, using scientific notation.
1.2 Calculate the charge on each sphere after separation.
Answers:
1.1
- A: +6,4 × 10–9 C
B: –24,6 × 10–9 C.
For scientific notation only one figure must be before the decimal comma, so B: –2,46 × 10–8 C.
1.2
- Q = (Q1 + Q2) / 2
= [(+6,4 × 10–9) + (-2,46 × 10–8)] / 2
= 9,1 × 10–9 C
2. A glass rod is charged positively by rubbing it with a silk cloth. The charge on the rod is 1,12 × 10 –11C. Calculate the number of electrons that were rubbed off the glass rod.
Answer:
Q = nqe
n = Q / qe
= 1,12 × 10–11 / 1,6 × 10–19
= 7 × 107 electrons
3 Electric circuits
3.1 Potential difference
- An electric current is a flow of charge, positive or negative.
- A battery supplies electrons to a circuit at its negative terminal and draws them in at the positive terminal by means of a chemical reaction in the battery. So, there is a conversion of chemical potential energy in the battery to electrical potential energy of the electrons.
- The voltage measured across the terminals of a battery when it is not providing current to a circuit is called the emf of the battery.
- The voltage measured across the terminals of a battery when it is providing current to a circuit is called the potential difference across the circuit. This is always smaller than the emf, due to the fact that the battery has some resistance.
- Emf and potential difference are measured in volts with a voltmeter, which has a very high resistance and is always connected in parallel across the circuit or resistor.
3.2 Current
- Current (I) is the rate of flow of charge.
- I = Q/Δt
- While an electric current can be a flow of negative or positive charge, current direction is shown as the direction in which positive charge would move in the circuit – from positive to negative. This is referred to as ‘conventional current’.
- Current is measured in amperes (A). 1 ampere = 1 coulomb per second
- Definition of an ampere: The current in a conductor is one ampere when one coulomb of charge passes through the conductor per second.
- I = Q/Δt
- Current is measured with an ammeter, which has a very low resistance and is always connected in series.
3.2.1 Worked example
Calculate the total charge that passes through a light bulb in 2 minutes when the current is 4 amperes.
Answer:
Q = I × Δt (Δt must be expressed in seconds: 2 minutes = 120 s)
= 4 × 120
= 480 C
3.3 Resistance
- Resistance (R) is the extent to which a resistor limits the flow of charge in it. When connected to the same potential difference, the higher the resistance of the resistor, the smaller the current.
- Resistance is measured in ohms (Ω).
- Definition of an ohm: A resistor has a resistance of 1 ohm if it allows a current of 1 ampere when the potential difference across it is 1 volt. So, an ohm is a volt per ampere.
- Factors affecting resistance: Resistance depends on the type of metal, length, thickness and temperature.
- A metal will have a higher resistance if its outer electrons are held more tightly by the nucleus of the atom. So, for instance, nichrome (an alloy of nickel and chromium) has a much higher resistance than copper.
- Current in metals is a flow of loosely bound electrons in the metal. The battery sets up an electric field in the circuit. The loosely bound electrons move under the action of this field. There is a conversion of electrical potential energy into kinetic energy of the moving electrons. The electrons collide with the atoms of the metal, causing them to vibrate faster. So, there is a conversion of kinetic energy of the electrons to
vibrational kinetic energy of the atoms in the metal. The faster the atoms vibrate, the hotter they are. If very hot, they could give out light. - Energy conversions:
- Chemical potential energy in battery electrical potential energy of electrons kinetic energy of electrons vibrational kinetic energy of atoms of metal heat energy and possibly light energy.
- Resistance increases as the length of the resistor increases.
- Resistance decreases as the thickness of the resistor increases.
- Resistance increases as the temperature of the resistor is increased.
- When a resistor is added in series to an identical one, the total resistance is doubled.
- When a resistor is added in parallel to an identical one, the total resistance is halved.
- To calculate the total resistance (equivalent resistance) Rs of a number of resistors connected in series, simply add them together:
Rs = R1 + R2 + R3 …… - Current is the same at all points in a series circuit.
- Resistors in series divide the potential difference in proportion to the resistance. They are voltage dividers. Add the potential differences across each resistor together to get the potential difference across the whole circuit.
- Resistors in parallel divide the current – they are current dividers. Add the currents together to get the mainstream current.
- The voltage across each resistor in a parallel connection is the same.
- To calculate the total resistance (equivalent resistance) Rp of a number of resistors in parallel, apply the formula:
1 / Rp = 1 / R1 + 1 / R2 + 1 / R3 …..
After calculating the right-hand side of the equation, remember to invert both sides. - For two resistors in parallel, the equation can be written as:
Rp = R1R2 / (R1 + R2) (remember: product ÷ sum) - Remember that this equation can only be used for two resistors in parallel.
3.1.1 Worked examples
- In the circuit diagram below, what is:

1.1 the total resistance?
1.2 the reading on voltmeter V?
1.3 the reading on ammeter A?
Answers:
1.1
- Rs = R1 + R2
= 2 + 1
= 3 Ω
1.2 The two resistors divide the voltage of the battery. If one voltmeter reads 4 V, the other must read 2 V.
1.3 Current is the same at all points in a series circuit, so the ammeter reads 2 A.
2 In the circuit diagram below, what is: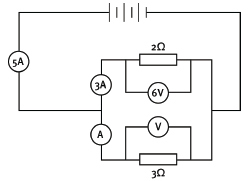
2.1 the equivalent resistance of the two resistors in parallel?
2.2 the reading on ammeter A?
2.3 the reading on voltmeter V?
Answers:
2.1
- 1 / Rp = 1 / R1 + 1 / R2 OR Rp = R1R2 / (R1 + R2)
= 1 / 2 + 1 / 3 OR = 2 × 3 / (2 + 3)
= (3 + 2) / 6 OR = 6 / 5
Rp = 6 / 5 = 1,2 Ω OR = 1,2 Ω
2.2 Resistors in parallel divide the current. The mainstream current is 5 A, and the current in one branch is 3 A. So the ammeter A must read 2 A.
2.3 The voltage must be the same as the voltage across the 2 Ω resistor. So the voltmeter V reads 6 V.
Waves, Sound and Light Questions and Answers Grade 10
Questions
Question 1: Multiple choice
Choose the correct answer. Write down only the letter of the answer you select.
1.1 What is the angle between the electric and magnetic fields in an electromagnetic wave?
- 0°
- 90°
- 180°
- 360° (3)
1.2 What is the period of a wave with a frequency of 5 Hz?
- 0,2 s
- 5,0 s
- 2,0 s
- 20,0 s (3)
1.3 Two transverse pulses meet and cancel out through a process called:
- diffraction.
- reflection.
- constructive interference.
- destructive interference. (3)
1.4 In order to calculate the speed of a wave, which formula would you use?
- wavelength ÷ frequency
- frequency ÷ wavelength
- wavelength ÷ period
- frequency × period (3)
1.5 The speed of a water wave is 4 m.s-1. If the frequency is 8 Hz, what is the wavelength?
- 32 m
- 2 m
- 12 m
- 0,5 m (3)
1.6 A vibrating hacksaw blade completes 40 oscillations (complete vibrations) in 5 s. What is its period?
- 8 s
- 0,125 s
- 0,2 s
- 0,025 s (3)
1.7 The speed at which water molecules are moving in a wave in a ripple tank:
- is greatest in a trough.
- is greatest in a crest.
- is smallest in the rest position.
- is greatest in the rest position. (3)
1.8 Sound travels fastest through a:
- solid.
- liquid.
- gas.
- vacuum. (3)
1.9 The sketch shows a rope with two pulses of equal amplitude approaching each other. When the two pulses pass through point X, what is the maximum ampli- tude of the pulse?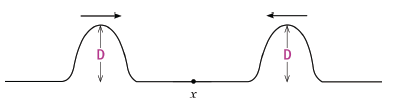
- D
- 0
- 2 D
- ½ D (3)
1.10 Which diagram below has both the wavelength () and the amplitude (A) la- belled correctly?



 (3)
(3)
1.11 Sound is:
- a series of moving compressions and rarefactions.
- an example of a transverse wave.
- able to travel through a vacuum.
- an example of a longitudinal wave.
Which of the above statements about sound is/are correct?
- 1, 2 and 3
- 1 and 2 only
- 1 and 3 only
- 1 and 4 (3)
1.12 The wavelength of a particular form of electromagnetic radiation in a vacuum is 10–12 m. The wavelength of a form of electromagnetic radiation of twice the frequency is:
- 2,5 × 10–13 m.
- 10–6 m.
- 5 × 10–13 m.
- 2 × 10–12 m. (3)
1.13 The energy of a photon of electromagnetic energy can be calculated using the equation:
- E = hf.
- E = h ÷ f.
- E = λ ÷ hc.
- E = hλ ÷ c. (3) [39]
Question 2: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
2.1 In a longitudinal wave, each particle in the medium is travelling fastest as it passes through the rest position. (2)
2.2 In a transverse wave, a pulse length is equal to a wavelength. (2)
2.3 Wavelength is the maximum displacement from the position of rest. (2)
2.4 An increase in frequency of a sound wave and a simultaneous increase in amplitude will cause a note that is louder and has a lower pitch. (2)
2.5 The energy of electromagnetic radiation is directly proportional to the wavelength of the radiation. (2) [10]
Question 3: One-word answers
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
3.1 The colour of visible light that has the shortest wavelength, highest frequency and greatest energy. (1)
3.2 A region in a longitudinal wave where the particles of the medium have been pulled far apart. (1)
3.3 The type of electromagnetic radiation that is responsible for us feeling the heat from the sun. (1)
3.4 The distance between two consecutive points in a longitudinal wave that are in phase. (1)
3.5 A quantum of visible light. (1) [5]
Question 4: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
4.1 released during nuclear reactions | A S |
4.2 a unit for frequency | B electric field |
4.3 a charged particle experiences a force | C gamma rays |
4.4 distance moved per second | D speed |
4.5 a quantum of visible light | E s–1 |
F photon | |
G magnetic field | |
H microwaves | |
I proton | |
J frequency |
[5]
Question 5: Long questions
The sketch below shows a pendulum consisting of a weight attached to the end of a length of string. The pendulum was set in motion by pulling the weight to position A and releasing it.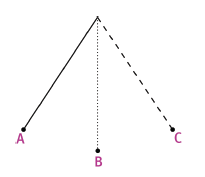
Explain the meaning of each of the following terms, making use of the positions shown in the sketch where appropriate.
5.1 one oscillation (or complete vibration) (2)
5.2 the rest position (or equilibrium position) (2)
5.3 frequency (2)
5.4 amplitude (2)
5.5 period (2) 5 × 2 = [10]
Question 6: Long questions
The sketch below shows a transverse wave in a medium.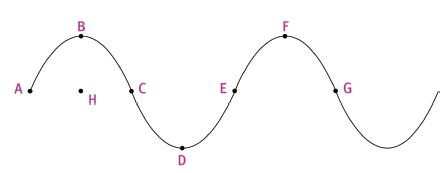
Use the letters supplied in the sketch to indicate the following:
6.1 the rest position (position of equilibrium) (2)
6.2 two points that are in phase (2)
6.3 a crest 2)
6.4 amplitude (2)
6.5 two points completely out of phase (2)
6.6 wavelength (2) 6 × 2 = [12]
Question 7: Long questions
Refer again to the illustration in question 6. Ten wavelengths pass point B in 2 seconds. The distance between points B and F is 300 mm. Calculate (in SI units):
7.1 the frequency (2)
7.2 the period (3)
7.3 the speed of the waves. (4) [9]
Question 8: Long questions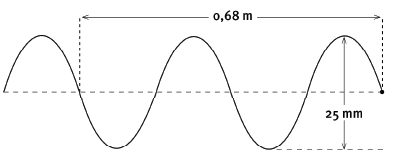
Each particle in the wave shown above completes one vibration (oscillation) in 0,4 s.
8.1 How long will it take eight wavelengths to pass a specific point in the medium? (2)
8.2 What is the amplitude of this wave? (2)
8.3 What is the period of this wave? (2)
8.4 What is the wavelength of this wave? (2)
8.5 Calculate the frequency of the wave. (3)
8.6 Calculate the speed of the wave. (4) [15]
Question 9: Long questions
Two beads are attached to the vibrator of a ripple tank and are positioned so that they dip into the water, as shown in the photo above. The resultant pattern is shown in the second photograph.
9.1 What is the phenomenon in the tank called? (2)
9.2 What do the fan-shaped lines in the second photo represent? (2)
9.3 How are these lines formed? (2) [6]
Question 10: Long questions
10.1 What type of wave is a sound wave that reaches your ear? (2)
10.2 Explain briefly how you would use a slinky spring to demonstrate this type of wave to a friend. (4)
10.3 What is meant by the ‘wavelength’ of this type of wave? (2) [8]
Question 11: Long questions
A typical sound wave associated with human speech has a frequency of 500 Hz, while the frequency of yellow light is about 5 × 1014 Hz. Assuming that sound travels at 340 m.s-1 and light at 3 × 108 m.s–1:
11.1 Calculate the wavelength of the sound wave. (4)
11.2 Calculate the wavelength of yellow light. (3)
11.3 Express the wavelength of yellow light in nanometres. (1) [8]
Question 12: Long questions
2.1 Describe an experiment or demonstration that shows that sound cannot travel in a vacuum. (5)
12.2 Why is the moon sometimes referred to as ‘the silent planet’? (2)
12.3 The speed of sound in air is 340 m.s–1. Calculate the wavelength of the sound produced by a tuning fork of frequency 156 Hz. (4)
12.4 Calculate the period of this sound wave. (3)
12.5 What is the sound called that has a frequency higher than the human ear can hear? (1)
12.6 Describe one use for this type of high-frequency sound wave. (2) [17]
Question 13: Long questions
Vibrations of frequency 2,0 Hz are produced by a generator attached to a spring. These vibrations are at 90° to the spring. The waves that it produces have a wavelength of 0,45 m.
13.1 What type of wave is passing along the spring? (1)
13.2 How many complete wavelengths pass a point in the spring in 3 seconds? (2)
13.3 What is the speed of the waves along the spring? (4)
13.4 Calculate the time taken for three complete wavelengths to pass a point in the spring. (5)
13.5 What is the wavelength of the waves along the spring if their frequency is in- creased to 6,0 Hz, without changing the tension (stretch) of the spring? (4) [16]
Question 14: Long questions
Sunlight is a form of electromagnetic radiation.
14.1 What is an ‘electromagnetic wave’? (3)
14.2 What causes electromagnetic waves? (2)
14.3 What is the speed of all electromagnetic waves in a vacuum? (1)
14.4 Assume that the sun is 1,5 × 108 km from the Earth. Calculate the time taken for sunlight to travel to the Earth. (5) [11]
Question 15: Long questions
A gamma ray has a period of 2 × 10–24 s.
15.1 What is a gamma ray? (2)
15.2 Why is it dangerous for humans to be exposed to gamma rays? (2)
15.3 What is the frequency of this gamma ray? (3)
15.4 Calculate the wavelength of the gamma ray in metres. (4) [11]
Question 16: Long questions
16.1 What types of radiation are A and B? (2)
16.2 Which type of radiation can cause tanning of the skin? Give another use for this type of radiation. (4)
16.3 Which type of radiation next to A in the spectrum has a longer wavelength than A? Give one use for this type of radiation. (3) [9]
Question 17: Long questions
Max Planck proposed that there is a relationship between the energy of a quantum of electromagnetic radiation and the frequency of the wave.
17.1 What is meant by a ‘quantum’ of electromagnetic radiation? (2)
17.2 What is a quantum of visible light called? (2)
17.3 What is the relationship between E and f as proposed by Max Planck? (2)
17.4 If we were to draw a graph of the energy of a quantum vs. the frequency of the quantum, what would the shape of the graph be? (2) [8]
Question 18: Long questions
Calculate the energy content of a quantum of each of the following types of electromagnetic radiation:
18.1 a radio wave of frequency 600 kHz (4)
18.2 a green light wave of wavelength 500 nm in air (5)
18.3 an X-ray of wavelength 12 pm in air (5) [14]
Question 19: Long questions
In a totally dark room, the human eye is only able to detect a flash of red light if the flash consists of at least 50 photons and if the flash is directed straight into the eye. Red light has a wavelength of 450 nm. Calculate the total minimum energy of a flash of red light that can be detected by the human eye. [7]
Answers
1.1 B
1.2 A
1.3 C
1.4 C
1.5 D
1.6 B
1.7 D
1.8 A
1.9 C
1.10 C
1.11 D
1.12 C
1.13 A
2.1 True
2.2 False. In a transverse wave, a pulse length is half as long as a wavelength. OR In a transverse wave, a wavelength is twice as long as a pulse length.
2.3 False. Amplitude is the maximum displacement from the position of rest. OR Wavelength is the distance between two consecutive points that are in phase.
2.4 False. An increase in frequency of a sound wave and a simultaneous increase in amplitude will cause a note that is louder and has a higher pitch.
2.5 False. The energy of electromagnetic radiation is directly proportional to the frequency of the radiation.
3.1 violet
3.2 rarefaction
3.3 infrared
3.4 wavelength
3.5 photon
Question 4: Matching pairs
4.1 C
4.2 E
4.3 B
4.4 D
4 5 F
Question 5: Long questions
5.1 movement from B to C to B to A and back to B
5.2 position B
5.3 number of oscillations per second
5.4 horizontal distance from B to A (or B to C)
5.5 time taken for one oscillation
Question 6: Long questions
6.1 line through points AHCEG
6.2 A and E (or B and F) (or C and G)
6.3 B (or F)
6.4 distance BH
6.5 A and C (or B and D) (or C and E) (or D and F) (or E and G)
6.6 straight-line distance AE (or BF) (or CG)
Question 7: Long questions
7.1
- Frequency is the number of wavelengths that pass a point per second. If 10 pass in 2 seconds, 5 must pass in one second.
f = 5 Hz
7.2
- T = 1 / f
= 1 /5
= 0,2 s
7.3
- Distance BF is one wavelength.
λ = 300 mm = 0,3 m
v = f λ
= 5 × 0,3
= 1,5 m.s–1
Question 8: Long questions
8.1 One oscillation of a particle produces one wavelength. So one wavelength passes a point in 0,4 s. Eight wavelengths pass in
- 8 × 0,4 s = 3,2 s.
8.2 Amplitude is distance from rest position to crest = 12,5 mm = 0,0125 m.
8.3 0,4 s
8.4
- The given distance is for 2 wavelengths.
λ = 0,68 / 2 = 0,34 m
8.5
- f = 1 / T
= 1 / 0,4
= 2,5 Hz
8.6
- v = f λ
= 2,5 × 0,34
= 0,85 m.s–1
Question 9: Long questions
9.1 constructive and destructive interference
9.2 flat water (or areas of destructive interference)
9.3 These are areas where crests and troughs meet, and cancel out to produce flat water.
Question 10: Long questions
10.1 longitudinal wave
10.2 Fix one end of the spring or have the friend hold it still. Stretch the spring. Hold the other end of the spring and push it forward and backwards rapidly and continuously along the straight line of the spring.
10.3 It is the distance between two consecutive crests (or two consecutive troughs or two consecutive points that are in phase).
Question 11: Long questions
11.1
- v = f λ
λ = v / f
= 340 / 500
= 0,68 m
11.2
- λ = v / f
= 3 × 108 / 5 × 1014
= 6 × 10–7 m
11.3 6 × 10–7 m = 600 nm
Question 12: Long questions
12.1 For the demonstration you will need an electric bell or buzzer with a switch, a suitable power supply, and a glass jar that can be connected to a vacuum pump that is able to pump the air out of the jar to produce a near vacuum. Connect the bell or buzzer to the power supply. Place it in the jar and switch on. You will hear the bell ringing loudly. Switch on the vacuum pump to extract air. The sound gets fainter and fainter. In a total vacuum, the sound would be inaudible.
12.2 The moon does not have an atmosphere, so no sound can be heard on the moon.
12.3
- v = f λ λ = v = 340
f 156
= 2,18 m
12.4
- T = 1 = 1
f 256
= 3.9 × 10–3 s (or 0,0039 s)
12.5 Ultrasound
12.6 Used in medicine to observe internal organs such as a baby in the womb. OR Used in industry to detect cracks in metals.
Question 13: Long questions
13.1 Transverse wave
13.2 f = 2 Hz, so two wavelengths pass a point in one second. Therefore, in three seconds, six wavelengths pass a point.
13.3
- v = f λ
= 2 × 0,45
= 0,9 m.s–1
13.4
- T = 1 / f
= 1 /2
= 0,5 s
One wavelength passes in 0,5 s, so three wavelengths pass in 1,5 s.
13.5 If the tension of the spring does not change, speed of the wave is constant. Frequency and wavelength are inversely proportional to each other. So, if frequency is made three times larger, wavelength must be made three times smaller. So the wavelength is 0,15 m.
OR
- λ = v / f
= 0,9 / 6
= 0,15 m
Question 14: Long questions
14.1 A changing electric field produces a changing magnetic field, which in turn produces a changing electric field. An electromagnetic wave is a transverse wave consisting of electric and magnetic fields at 90° to each other. The crests and troughs represent points where the electric or magnetic fields are strongest.
14.2 Accelerating charges produce electromagnetic pulses. A continuous electromagnetic wave is produced by vibrating charges, as in alternating current.
14.3 3 × 108 m.s–1
14.4
- First convert to SI units. 1,5 × 108 km = 1,5 × 1011 m
time = distance / speed = 1,5 × 1011 / 3 × 108
= 500 s (This is 8 minutes and 20 seconds.)
Question 15: Long questions
15.1 Gamma rays are very high-frequency and high-energy electromagnetic radiation emitted by radioactive material.
15.2 They can destroy human tissue and cause cancer.
15.3
- f = 1 / T
= 1 / 2 × 10–24
= 5 × 1023 Hz
15.4
- c = f λ
λ = c / f
= 3 × 108 / 5 × 1023
= 6 × 10–16 m
Question 16: Long questions
16.1
- A – infrared
- B – X-rays
16.2
- ultraviolet
- Certain chemicals fluoresce in ultraviolet light. For example, chemicals in washing powder will fluoresce under the ultraviolet light from the sun, making garments look whiter than they actually are.
16.3
- microwaves
- In microwave ovens, water in the foodstuffs are made to vibrate faster by the microwaves, thereby getting hotter.
Question 17: Long questions
17.1 ‘Quantum’ means a discreet amount or ‘package’. So, the radiation is not in con- tinuous waves, but small packages of energy, each made up of electromagnetic waves.
17.2 photon
17.3 E is directly proportional to f. This means that if f is doubled, E is doubled.
17.4 a straight line through the origin
Question 18: Long questions
18.1
- f must be expressed in Hz. 600 kHz = 6 × 105 Hz
E = hf
= 6,63 × 10–34 × 6 × 105
= 3,98 × 10–28 J
λ must be expressed in metres. 500 nm = 500 × 10–9 m
E = hc / λ
= (6,63 × 10–34) × (3 × 108)
500 × 10–9
= 3,98 × 10–19 Hz
λ must be expressed in metres. 120 pm = 120 × 10–12 m
E = hc / λ
= (6,63 × 10–34) × (3 × 108)
120 × 10–12
= 1,66 × 10–15 J
Question 19: Long questions
First calculate the energy of one photon of red light. 450 nm = 450 × 10–9 m
- E = hc /λ
= (6,63 × 10–34) × (3 × 108)
450 × 10–9
= 4,42 × 10–19 J
Minimum number of photons = 50
Minimum energy of the flash = (4,42 × 10–19) × 50
= 2,21 × 10–17 J
Waves, Sound and Light - Physical Science Grade 10 Study Guide
Overview
Summary
1 Transverse pulses and waves
1.1 Properties of transverse pulses and waves
- A pulse is a single disturbance in a medium. A single crest is a transverse pulse. A single trough is also a transverse pulse.
- In a transverse pulse or wave, the particles of the medium vibrate at 90° to the direction in which the pulse or wave moves.
- The amplitude of a pulse is the maximum displacement from the position of rest of a particle in the medium.
- A wave is made up of one pulse after another.
- The ‘hump’ in a transverse wave is called a crest.
- The ‘hollow’ in a transverse wave is called a trough.
- Continuous transverse waves are produced by continuous vibrations of the medium.
- A vibration is a regular to-and-fro movement (up-and-down or forwards-and- backwards).
- The rest position of a vibrating object (also called the equilibrium position) is the position that it would be in when not vibrating.
- One complete vibration (also called one oscillation) is one complete to-and-fro movement. It is the movement from the rest position to the furthest point in one direction, then to the furthest point in the opposite direction, then back to the rest position.
- One complete vibration (or one oscillation) of the end of a slinky spring will produce one wavelength in the spring.
- Particles in a medium are in phase if they are vibrating perfectly in step with one another.
- Particles in a medium that are not vibrating perfectly in step with one another are out of phase. Two particles are completely out of phase if they are moving oppositely, with one reaching the crest at the same instant that the other reaches the trough.
1.2 Wavelength, frequency, amplitude, period, wave speed
- Wavelength (λ) is the distance between two consecutive points that are in phase. For transverse waves, wavelength is the distance between two successive crests or two successive troughs. The unit is metres (m). If the wavelength is given in any other unit (for instance, mm or nm), it must be converted to metres when doing a calculation.
- Frequency (f) is the number of wavelengths passing per second. It equals the frequency of the vibration making the waves. The unit is s–1 (per second). 1 s–1 is called a hertz (Hz).
- The amplitude of a wave is the maximum distance that a point in a wave moves from its rest position. This equals the distance from the rest position to the top of a crest or to the bottom of a trough. The unit is metres (m).
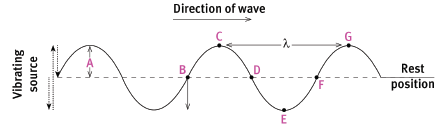
Distance A is the amplitude of the wave. Each point in the medium simply vibrates up and down. Point B must be moving down for the wave to be moving to the right. Point B and F and points C and G are in phase. Points B and D and points C and E are completely out of phase. Distance CG and distance BF are equal and are the wavelength.
- The speed of a wave (v) is the distance moved by any pulse in the wave per second. The speed can change as the medium changes. The unit is metres per second (m.s–1). If the speed is given in any other unit (for instance, km hr–1), it must first be converted to m.s–1 before doing a calculation.
- Speed is calculated using the formula: speed (in m.s–1) = Distance moved (in m)
time taken (in s) - The wave equation relates the above three quantities: v = f λ
- The period (T) of a wave is the time taken for one wavelength to pass. The unit is seconds (s). T = 1 / f
- Period and frequency are inversely proportional to each other. If the frequency is doubled, the period is halved. It also equals the period of the vibration making the wave.
- If T = 1 / f , it follows that f = 1 / T.
- If we take the wave equation v = f λ and substitute 1 / T for f, we have v = λ / T.
1.2.1 Worked example
A transverse wave is set up in a slinky spring lying on a long table. The wavelength is 540 mm. One wavelength passes a mark on the table every 0,8 s.
Calculate:
- the frequency of the wave
- the speed of the wave.
Answers:
- T = 0,6 s f = ?
f = 1 = 1
T 0,8
= 1,25 Hz - f = 1,25 Hz λ = 540 mm = 0,54 m v = ?
v = f λ
= 1,25 × 0,54
= 0,675 m.s–1
1.3 Superposition of pulses
- Superposition is the addition of the amplitudes of two pulses that occupy the same space at the same time. If a crest is considered positive, then a trough is negative.
- When waves meet, they interfere.
- Crest meeting crest or trough meeting trough results in a bigger amplitude – constructive interference.
- Crest meeting trough results in a smaller amplitude – destructive interference.
- Two pulses will cancel out to produce zero amplitude only if:
- one is a crest, the other a trough
- their amplitudes are equal
- their pulse lengths are equal.
2 Longitudinal waves and sound
2.1 Longitudinal pulses and waves
- A pulse is a single disturbance in a medium. A single compression (particles close together) or a single rarefaction (particles far apart) are each longitudinal pulses.
- The amplitude of a pulse is the maximum displacement from the position of rest of a particle in the medium. So it is the distance from the rest position to the centre of a compression, or the distance from the rest position to the centre of a rarefaction.
- In a longitudinal wave, the particles of the medium vibrate in line with the direction in which the wave moves.
- Longitudinal waves are made up of alternate compressions (particles close together) and rarefactions (particles far apart).
- Wavelength (λ) is the distance between two consecutive points that are in phase. For longitudinal waves, wavelength is the distance between two successive compressions or two successive rarefactions. The unit is metres (m). If the wavelength is given in any other unit (for instance, mm or nm), it must be converted to metres when doing a calculation.
- Frequency (f) is the number of wavelengths passing per second. It equals the frequency of the vibration making the waves. The unit is s–1 (per second). 1 s–1 is called a hertz (Hz).
- The amplitude of a wave is the maximum distance that a point in a wave moves from its rest position. For a longitudinal wave, it is the distance from the rest position to the centre of a compression or to the centre of a rarefaction. The unit is metres (m).
- The period and frequency of a longitudinal wave have the same meaning as for a transverse wave. Period is the time taken for one wavelength to pass, for instance, the time between two successive compressions, measured in seconds. Frequency is the number of wavelengths that pass per second, measured in Hz.
- The equations T = 1 / f and v = f λ are applied as for transverse waves.
2.2 Sound waves
- Only vibrating objects produce sound. Energy is therefore needed to produce sound.
- A material medium is required for sound to move. Sound cannot pass through a vacuum.
- Sound energy moves through a medium as longitudinal waves. Alternate compressions and rarefactions pass through the medium.
- The speed, frequency and wavelength of sound are related by the equation:
Speed = frequency × wavelength
v = f λ - The speed of sound in air is approximately 340 m.s–1. The speed is dependent on the medium and its temperature.
- Solids transmit sounds best, gases worst. The same sound is heard loudest through solids, next loudest through liquids and softest through gases.
- Sound moves fastest through solids and slowest through gases. In gases, the greater the mass of the gas molecules, the slower the sound. In air, the higher the temperature the faster the sound.
2.3 Pitch and loudness
- The frequency of a sound wave is determined by the frequency of the vibration that causes it. If the sound wave passes into another medium, say from air into water, the speed of the wave changes, but the frequency stays the same.
- The pitch of a note is its position on a musical scale. As the frequency of a sound wave increases, so the pitch rises.
- Loudness is determined by the amplitude of the sound wave. The greater the amplitude, the louder the sound.
- Ultrasound has frequencies between 20 kHz and 100 kHz, higher than the range of human hearing. Ultrasound is used to produce internal images of the body (for instance, of a baby in the womb). The sound is reflected differently by the different layers in the body.
3 Electromagnetic radiation
3.1 Wave nature and spectrum
- The spectrum of visible light is only a small part of a broad range of waves that travel through a vacuum at the speed of light. The full range is called the electromagnetic spectrum.
- Electromagnetic waves are produced by accelerating charges. For example, in a radio aerial, changing electric fields accelerate electrons back and forth. This produces a changing magnetic field at right angles to the aerial. This in turn produces changing electric fields at right angles, and the process continues with each field generating the other. The crests and troughs in the wave indicate points where the electric or magnetic fields are strongest.
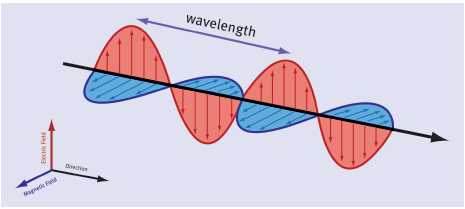
- Electromagnetic waves are transverse and consist of changing electric fields and magnetic fields at 90° to each other.
- Electromagnetic waves travel through space at 3 × 108 m.s–1. The wave equation c = f λ applies.
- Different sections of the spectrum have different names. In order of increasing wavelength (or decreasing frequency), these are: gamma rays, X-rays, ultraviolet light, visible light, infrared, microwave, TV and radio.
- Gamma rays are produced by radioactive material. They have the highest frequency and the highest energy and are the most penetrative and the most dangerous. They can destroy human cells and cause cancer. Radio waves at the opposite end of the spectrum are far less penetrative and not dangerous.
- X–rays are produced when high-speed electrons strike a metal plate. They can be used to produce a photographic image of the human body. They cannot pass through lead.
- Ultraviolet radiation is produced by very hot objects. Ultraviolet rays are very harmful to the eyes, cause tanning and can cause skin cancer. Some chemicals fluoresce in UV light.
- Infrared radiation is heat radiation, produced by vibrating atoms and molecules in hot objects. TV remotes work by sending out infra-red pulses.
3.2 The wave and particle nature
- Electromagnetic waves are radiated in packages, called quanta. A quantum of light is called a photon.
- Electromagnetic radiation has both a wave nature (transverse electric and magnetic fields) and a particle nature (quanta of energy). Radio waves have such long wavelengths that their particle nature is negligible. Gamma rays have such short wavelengths that their wave nature is negligible. Visible light (in the middle of the electromagnetic spectrum) behaves both as waves and as particles. This is known as the dual nature of light.
- The energy of a quantum can be calculated using the equation:
E = h f = h c / λ
where h = 6,63 × 10–34 J.s (Planck’s constant) and c = 3 × 108 m.s–1
3.2.1 Worked Examples
- The energy of a photon of light is 5,3 × 10–19 J. Calculate the frequency of the light waves.
- Calculate the energy of a microwave quantum of wavelength 0,2 m.
Answers:
- E = 5,3 × 10-19
E = h f f = E / h = 5,3 × 10-19 / 6,63 × 10–34
f = 7,99 × 1014 Hz - E = hc / λ = (6,63 × 10–34) × (3 × 108)
0,2
E = 9,95 × 10–25 J
Matter and Materials Questions and Answers Grade 10
Questions
Question 1: Multiple choice
Choose the correct answer. Only write the letter of the answer you select.
1.1 Where are metals found on the periodic table?
- At the bottom
- To the right
- To the left
- At the top (3)
1.2 What is the name given to the group VII elements?
- Alkali metals
- Alkaline earth metals
- Halogens
- Nobel gases (3)
1.3 What change to a neutral atom will result in the formation of a negative ion?
- It gains an electron.
- It gains a proton.
- It loses an electron.
- It loses a proton. (3)
1.4 Which statement about the numbers of particles in atoms is correct? Apart from hydrogen, most atoms contain:
- more neutrons than protons.
- more protons than neutrons.
- more electrons than protons.
- more protons than electrons. (3)
1.5 Metal atoms form:
- positive anions.
- negative anions.
- negative cations.
- positive cations. (3)
1.6 Which are the correct formulae for sodium chloride and calcium carbonate?
- NaCl and CaCO3
- SCl and CaCO3
- NaCl and CaCO2
- NaCl and CmCO3 (3)
1.7 Which of the elements below has an electron configuration of 1s22s22p4?
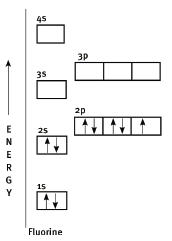
- sodium
- chlorine
- oxygen
- fluorine (3)
1.8 Which of the following terms describes the change in state that occurs when a liquid changes into a solid?
- condensation
- evaporation
- freezing
- sublimation (3)
1.9 Copper has two isotopes; 69,1% of copper isotopes have a mass of 63 and 30,9% have a mass of 65. What is the average mass of a copper atom?
- 65
- 66
- 64,4
- 63,6 (3)
1.10 In which of the following compounds are electrons shared between atoms?
- sodium fluoride
- nitrogen dioxide
- iron bromide
- 1 only
- 2 only
- 1 and 3
- 1, 2 and 3 (3)
1.11 Which compound contains two double bonds in which electrons have been shared?
- hydrogen bromide
- carbon dioxide
- sodium iodide
- water (3)
1.12 In the molecules CH4, HBr and H2O, which atoms use all of their outer shell electrons in bonding?
- C and Br
- C and H
- Br and H
- H and O (3)
1.13 The following statement is about chemical bonding. Covalent bonds are formed by the … of electrons. Covalent bonds occur between ... Which combination of words completes the statement? (3)
A | transfer | two non-metals |
B | transfer | a non-metal and a metal |
C | sharing | two metals |
D | sharing | two non-metals |
1.14 Which of the elements below is most likely to form a positive ion?
- zinc
- chlorine
- oxygen
- fluorine (3)
1.15 Which substance when combined with oxygen will form a covalent bond?
- sodium
- magnesium
- boron
- aluminium (3) [45]
Question 2: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 formation of positive ions | A – the number of protons and neutrons added together |
2.2 mass number | B – loss of electrons |
2.3 group VIII elements | C – when a substance changes from a liquid to a solid state |
2.4 chromatography | D – the number of protons and electrons added together |
2.5 condensation | E – halogens |
F – gain of electrons | |
G – separation of mixtures of pigments/colours | |
H – when a substance changes from a gas to a liquid state | |
I – separation of a solid from a liquid | |
J – noble gases |
[5]
Question 3: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
3.1 Isotopes are elements with the same number of protons, but different numbers of electrons. (2)
3.2 Isotopes contain the same number of electrons in their outermost energy shell. (2)
3.3 Sodium has an electron configuration of 1s22s22p63s1. (2)
3.4 Calcium and magnesium both form anions with a charge of +2. (2)
3.5 A mixture can be separated into its component substances by physical means. (2) [10]
Question 4: One-word answers
Provide one word or term for each of the descriptions. Write only the word or term next to the question number.
4.1 The movement of particles from a region where there are many to a region where there are fewer. (1)
4.2 The basic building block of matter. (1)
4.3 The electrons found in the outermost energy shell. (1)
4.4 A mixture in which the different substances that make up that mixture can be seen. (1)
4.5 The type of chemical bonding that occurs when electrons are transferred from one atom to another. (1) [5]
Question 5: Long questions
Mixtures can be homogeneous or heterogeneous.
5.1 What is the difference between a homogeneous mixture and a heterogeneous mixture? (2)
5.2 Give one example of each. (2)
5.3 How do mixtures differ from compounds? (2)
5.4 Which of the following substance is pure?
- sugar
- sea water
- steel (1) [7]
Question 6: Long questions
The following are the melting points of the metals in group II on the periodic table:
- beryllium 1278 °C
- magnesium 649 °C
- calcium 839 °C
- strontium 769 °C
- barium 725 °C
6.1 What is the general trend in melting points as you go down the group? (1)
6.2 One metal does not fit this trend. Which one is it? (1)
6.3 Does this information support the idea that beryllium atoms are held together more strongly than barium atoms? (1)
6.4 Explain your answer to question 6.3. (2) [5]
Question 7: Long questions
Use the table below to answer the questions that follow.
Substance | Melting point (°C) | Boiling point (°C) |
lead | 317 | 174 |
radon | –71 | –62 |
ethanol | –117 | 78 |
cobalt | 1492 | 2900 |
nitrogen | –210 | –196 |
propane | –188 | –42 |
ethanoic acid | 16 | 118 |
7.1 Define the boiling point of a substance. (2)
7.2 Which two substances are gaseous at –50 °C? (2)
7.3 Which substance is a liquid at 2500 °C? (1)
7.4 Is nitrogen a liquid, solid or gas at 35 °C? (1) [6]
Question 8: Long questions
The table below shows information about two isotopes of chlorine.
Atom | Number of protons | Number of electrons | Number of neutrons |
Chlorine-35 | A | 17 | 18 |
Chlorine-37 | 17 | B | C |
8.1 Replace the letters with the correct numbers to complete the table. (3)
8.2 Define isotopes. (2)
8.3 Draw an Aufbau diagram of a chlorine atom. (2)
8.4 A chlorine ion has a charge of –1. Write down the electron configuration for a chlorine ion. (1) [8]
Question 9: Long questions
The diagram below shows the electron configuration of four different elements. 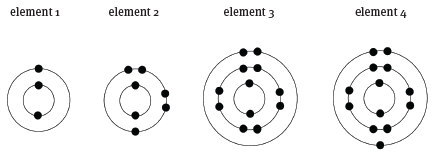
9.1 Which element has an atomic number of 3? (1)
9.2 Which atom has the electron configuration 1s22s2 2p63s2? (1)
9.3 Which element is nitrogen? (1) [3]
Question 10: Long questions
The structure of a typical ionic compound is a regular arrangement of positive and negative ions.
10.1 What is the name of this regular arrangement of particles? (1)
10.2 Name an ionic substance. (1)
10.3 Ions are formed by electron loss or gain.
10.3.1 Give the formula of the magnesium ion. (1)
10.3.2 Give the formula of the oxide (oxygen) ion. (1)
10.3.3 Why are these two ions attracted to each other? (1)
10.3.4 Draw a Lewis diagram to show the bonding that takes place between a magnesium and oxygen atom. (3) [8]
Question 11: Long questions
The table below describes the number of protons, neutrons and electrons found in three different substances: A, B and C.
Particle | Number of protons | Number of electrons | Number of neutrons |
A | 15 | 15 | 16 |
B | 15 | 18 | 16 |
C | 15 | 15 | 17 |
Use the information in the table to explain why the following statements are true.
11.1 Particle A is a neutral atom. (1)
11.2 They are all particles of the same element. (1)
11.3 Particle B is a negative ion. (1)
11.4 Particles A and C are isotopes. (2)
11.5 What is the charge on particle B? (1)
11.6 Is particle B a metal or a non-metal? Give a reason for your answer. (2) [8]
Answers to questions
Question 1: Multiple choice
1.1 C
1.2 C
1.3 A
1.4 B
1.5 D
1.6 A
1.7 C
1.8 C
1.9 D
1.10 B
1.11 B
1.12 B
1.13 D
1.14 A
1.15 C [45]
Question 2: Matching Pairs
2.1 B
2.2 A
2.3 J
2.4 G
2.5 H [5]
Question 3: True/False
3.1 False – Isotopes have the same number of protons, but different numbers of neutrons.
3.2 True
3.3 True
3.4 False – Sodium and calcium both form cations with a charge of +2.
3.5 True [10]
Question 4: One-word answers
4.1 diffusion
4.2 atom
4.3 valence electrons
4.4 heterogeneous
4.5 ionic [5]
Question 5: Long questions
5.1 In a homogeneous mixture, the substances that make up the mixture are not visible as separate substances whereas in a heterogeneous mixture they are.
5.2 Homogeneous – sea water, fruit juice, tea, coffee, steel (any correct one)
Heterogeneous – granite, nuts and raisins, smoke, oil and water, box of biscuits (any correct one)
5.3 In mixtures, the substances that make up that mixture can be in variable proportions. In compounds, the elements combine in fixed ratios. Mixtures can be separated by physical means, while compounds can only be separated by chemical means.
5.4 Only sugar is a compound. The rest are mixtures. [7]
Question 6: Long questions
6.1 melting points decrease
6.2 magnesium
6.3 yes
6.4 because the melting point of beryllium is much higher, so more energy is needed to melt it [5]
Question 7: Long questions
7.1 The boiling point of a substance is the temperature at which that substance changes from a liquid into a gas.
7.2 radon and nitrogen
7.3 cobalt
7.4 gas [6]
Question 8: Long questions
8.1 A 17
B 17
C 20
8.2 Elements with the same atomic number, but different mass numbers.
8.3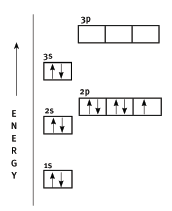
- correct number of electrons
● correct placement of electrons
8.4 1s22s22p63s23p6 [8]
Question 9: Long questions
9.1 element 1
9.2 element 3
9.3 element 2 [3]
Question 10: Long questions
10.1 crystal lattice
10.2 Any metal atom combined with a non-metal atom.
10.3 10.3.1 Mg+2
10.3.2 O–2
10.3.3 The electrostatic attraction between oppositely charged ions.
10.3.4
- correct number of valence electrons
- correct placement of valence electrons
- correct charges of ions [8]
Question 11: Long questions
11.1 It has the same number of protons as electrons.
11.2 They all have the same number of protons.
11.3 It has more electrons than protons.
11.4 They have the same number of protons, but different numbers of neutrons.
11.5 –3
11.6 It is a non-metal, as it has formed a negative ion. [8]
Matter and Materials - Physical Science Grade 10 Study Guide
Overview
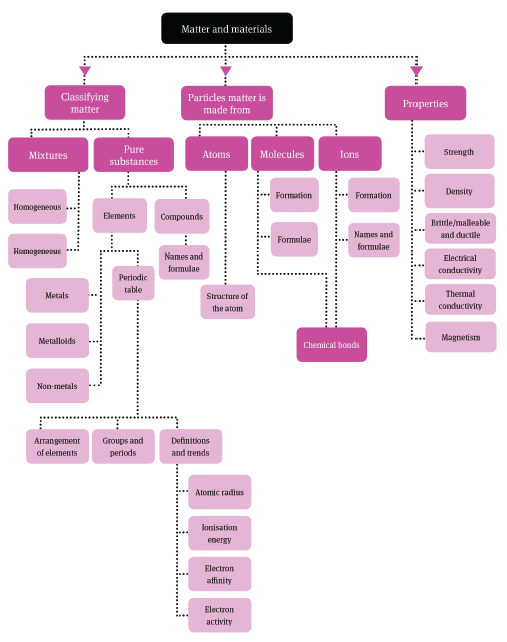
Summary
1 Revision of matter (Grade 9)
1.1 Matter and classification
- Matter is made up of particles (atoms or molecules) and it is the properties of the atoms or molecules that determine the characteristics and reactivity of that matter.
- The properties of matter include: strength; density; melting and boiling points; whether the material is brittle, malleable or ductile; whether it is magnetic or not; and its electrical and thermal conductivity.
- Elements and compounds are classified as pure because they contain particles that are all the same. Substances can be classified as pure – elements and compounds
– or mixtures. Pure substances contain particles that are all the same. Elements and compounds can be represented by symbols and formulae. Some of the names, symbols or formulae of a number of common elements and compounds that you should have learnt are the following:- Elements: S for sulphur; C for carbon; P for phosphorus (note the spelling); H for hydrogen; O for oxygen; He for helium; N for nitrogen; F for fluorine; Mg for magnesium; Ca for calcium; Zn for zinc.
- Compounds: H2O for water; H2SO4 for sulphuric acid; HCl for hydrochloric acid; NaCl for sodium chloride (table salt); CaCO3 for calcium carbonate; KNO3 for potassium nitrate; Na3PO4 for sodium phosphate.
- You will notice that the naming of compounds follows these rules:
- The metal or hydrogen is always named first, then the non-metal (potassium iodide: KI; hydrogen chloride: HCl (also called hydrochloric acid)).
- The name of a non-metal ion of an element always ends in –ide (for instance, sodium chloride: NaCl).
- The name of a non-metal complex ion (more than one element in the ion) usually ends in –ate or –ite (for instance, copper sulphate: CuSO4; potassium sulphite: K2SO3). An exception to this is hydroxide (for instance, sodium hydroxide: NaOH).
- Mixtures consist of different substances, and can be homogeneous (have the same ‘look’ throughout) or heterogeneous (you can see the different substances). They can be separated in various ways, for instance filtering, sieving, chromatography, distilling or dissolving one constituent.
- Some of the properties of metals are that they conduct electricity and heat, and they are malleable and ductile.
- Non-metals are non-conductors of electricity and heat. They are gases, liquids or brittle solids, and bond with each other.
- Metalloids are elements on the border between metals and non-metals, and they have both metal and non-metal properties.
2 States of matter and the kinetic molecular theory
- The kinetic molecular theory, together with an understanding of intermolecular forces, explains how matter can exist as solids, liquids or gases. The kinetic molecular theory can also be used to explain diffusion and Brownian motion.
- The kinetic molecular theory says that:
- Matter is made up of particles that are constantly moving.
- Particles have energy that varies according to whether they are in the gas (most energy), liquid or solid (least energy) phase.
- The temperature of a substance is a measure of the average kinetic energy of its particles.
- A change in phase may occur when the energy of the particles is changed by heating or cooling.
- There are spaces between the particles. These are greatest in the gas phase and least in the solid phase.
- Melting (solid → liquid or liquid → solid) points and boiling (liquid → gas or gas → liquid) points are the temperatures at which phase changes happen. They are also specific to pure substances.
- Sublimation is the change from solid to gas without going through the liquid phase, for instance, solid CO2 (dry ice) sublimates to CO2 (gas).
- Brownian motion is the random movement of gas and liquid particles. (‘Random’ means that any one particle can be moving in any possible direction at that instant.)
- Diffusion occurs mostly in gases and liquids. It is the movement of particles of one kind from an area where there are many to an area where there are few. This can happen because of the random movement of all particles and because there are large spaces between particles in the gas and liquid phases.
3 The atom – the basic building block of matter
3.1 The models of the atom
Matter is any substance that has a mass and occupies a volume. A number of models have been developed by different scientists explaining the structure of these atoms. Some of these models are described below:
- Democritus – developed the particle theory of matter. He stated that if you kept dividing something, eventually you would get to a point where it can no longer be divided. He called this indivisible substance ‘atomos’.
- Dalton – said all matter consists of atoms that cannot be made or destroyed. Atoms of the same element are all the same and atoms can be joined together.
- Thomson – developed the ‘currant bun’ model. An atom consists of a solid, positively charged mass in which tiny negatively charged particles are scattered.
- Rutherford – said an atom contains a central, positively charged mass known as the nucleus.
- Bohr – said negatively charged electrons are contained within certain areas in the atom known as ‘energy shells’.
- Schrödinger – developed the ‘wave model’ of the atom in which atoms behave as waves rather than as particles.
3.2 The structure of the atom
The atom consists of a central nucleus containing positively charged protons and neutral neutrons. Together these are known as nucleons.
Surrounding the nucleus is a number of energy shells containing negatively charged electrons.
The number of protons in the nucleus is called the atomic number (Z). In a neutral atom, the number of protons is equal to the number of electrons.
The number of protons and neutrons (added together) in the nucleus is called the mass number (A).
A = Z + N
Mass number Atomic number Number of neutrons
- An atom can either gain or lose electrons in order to form a charged particle, known as an ion. A positive ion is formed when an atom loses electrons, while a negative ion is formed when an atom gains electrons.
- The mass of an atom (atomic mass) is determined by the number of protons and neutrons. Mass of a proton = mass of a neutron = 1 mass unit.
3.3 Isotopes
- Isotopes are atoms of the same element that contain the same number of protons, but different numbers of neutrons. They have the same atomic number, but different mass numbers.
- In the periodic table, two numbers are given with the symbol of each element. These numbers are the atomic number and the average atomic mass.
- In any element, the ratio of the isotopes is constant. The atomic mass is given as the average mass of all the atoms in the sample of the element.
- We can use the percentage composition of the isotopes of an atom to determine the average mass of the element.
- Example: 75% of all naturally occurring chlorine atoms have an atomic mass of 35, while 25% have an atomic mass of 37. We can use these values to calculate the average atomic mass that will appear on the periodic table. We do this by multiplying the percentages of each isotope by their respective atomic masses and then dividing the answer by 100. Atomic mass of chlorine = [(75 × 35) + (25 × 37)] / 100 = 35,5.
3.4 Electron configuration
- Electron configuration refers to the arrangement of electrons in an atom. Electrons occupy orbitals arranged within energy shells (numbered 1, 2, 3 …) around the nucleus. These correspond to the periods in the periodic table.
- Orbitals are regions where electrons spend most of their time. Each orbital can contain a maximum of two electrons.
THE S-ORBITAL is spherical (ball-shaped).
EACH ENERGY shell has only one s-orbital.
P-ORBITALS ARE shaped like dumb-bells.
THERE ARE no p-orbitals in the first shell.
THE OTHER shells each contain three p-orbitals, named px, py and pz.
EACH ORBITAL can hold two electrons, which means that the three p-orbitals in a shell can hold six electrons in total.
- Scientists use Aufbau diagrams (arrows in boxes) to represent the electron configuration of an atom.
- The shell closest to the nucleus is number 1, the next number is 2, and so on.
Energy level | Types of orbitals | Maximum number of electrons contained |
1 | Only an s-orbital | 2 electrons in the s-orbital |
2 | An s- and 3 p-orbitals | 2 electrons in the s-orbital and 6 electrons in the p-orbitals |
3 | An s- and 3 p-orbitals | 2 electrons in the s-orbital and 6 electrons in the p-orbitals |
Example: An Aufbau diagram for fluorine
- Fluorine contains nine electrons. This means its electron configuration is 1s22s22p5. Each orbital must contain the maximum number of electrons before you can move on to the next orbital.

- As you can see, each electron is represented by an arrow.
- Rules for drawing Aufbau diagrams:
- You must always fill up the lower orbitals before you can move on to the next energy level.
- Pauli’s exclusion principle: ‘Each orbital may contain a maximum of 2 electrons, spinning oppositely’. This is why in each orbital the one arrow faces up, while the other faces down.
- Hund’s rule: ‘Electrons occupy equivalent orbitals (like the p-orbitals in a shell) singly, before pairing takes place’.
4 The periodic table
- The elements are arranged in order of increasing atomic number.
- The zig-zag (step) line separates the metals (on the left) from the non-metals (on the right).
- The numbered columns are called groups and are numbered from I to VIII. If the transition metals are included, then the numbers run from 1 to 18.
- The numbered rows are called periods. This number tells you how many energy levels or electron shells there are.
- In some groups the properties of the elements in the group are similar (for instance, the alkali metals of group I, the non-metal halogens of group VII)
- The properties change gradually as you move across a period (for instance, change from metal to non-metal).
- Characteristics that change across the periodic table include the following:
- Atomic radius: This is the distance from the centre of the nucleus to the outer energy shell, and it increases down a group and across a period.
- Ionisation energy: This is the energy needed to remove an electron from an atom of that element in the gas phase, and it increases across a period but decreases down a group.
- Electron affinity: This is the energy change when an electron is added to an atom of an element in the gas phase, and it increases across a period and down a group.
- Electronegativity: This is the ability of an atom to attract the electrons making a bond it is involved in, and it increases across a period but decreases down a group.
5 Chemical bonding
- The electrons found in the outermost energy shells are known as valence electrons and they control how an atom reacts.
- The number of valence electrons equals the group number.
- All atoms try to get a full outer shell of electrons. They can achieve this by gaining electrons from another atom, losing electrons to another atom or sharing electrons with another atom.
- Covalent and ionic bonding are shown using Lewis diagrams.
5.1 Lewis diagrams (dot-cross diagrams)
- Dots or crosses are used to represent the valence electrons of an atom.
- Rules for drawing Lewis diagrams:
- Write the symbol for the element and then draw a cross or dot on top of the symbol.
- Work in a clockwise direction.
- The electrons are only paired once the first four dots or crosses have been drawn.
5.2 Formation of ions
- An ion is a charged particle that forms when a neutral atom either gains or loses electrons.
- Atoms lose or gain electrons in order to obtain a full outer energy shell.
Group | Charge on the ion |
I | +1 |
II | +2 |
III | +3 |
IV | These elements do not form ions. |
V | –3 |
VI | –2 |
VII | –1 |
Elements in group VIII will not form ions, as they already have a full outer shell of electrons.
5.3 Ionic bonding
- Ionic bonding occurs between metals, which form positive ions, and non-metals, which form negative ions. Ionic bonding follows these rules:
- The metal will form a positive ion with a charge equal to the number of electrons it has lost.
- The non-metal ion is written in square brackets. Its charge is equal to the number of electrons gained.
- The square brackets must contain the transferred electrons as well as those of the non-metal ion.

5.4 Covalent bonding
- A covalent bond is formed by overlapping orbitals that share of a pair of electrons. In this way, both atoms obtain a full outer energy shell.
- Water is an example of a covalent compound. Each oxygen atom requires two electrons in order to obtain a full energy shell. This means that two hydrogen atoms are needed. In this case, two single bonds are formed.

- An oxygen molecule contains a double bond, as each oxygen atom shares two electrons. It can be written as O=O.
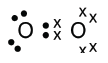
5.5 Metallic bonding
- The valence electrons of metals are loosely held by the nucleus. They move out of position, and become ‘delocalised’ or free electrons.
- The structure is held in place by the strong electrostatic force between the positively charged metal ions and the negatively charged delocalised electrons.
5 Particles that make up substances
6.1 Covalent molecular substances
- Molecules are formed when non-metal atoms are covalently bonded together.
- We can represent molecules by using different circles. Each atom is represented by a circle of a different colour and size.

6.2 Ionic substances
- Ionic substances, also known as salts, are formed when a metal atom transfers electrons to a non-metal atom.
- The electrostatic attraction between the positive and negative ions is what holds the crystal lattice together.
6.3 Covalent network structures
- Covalent network structures are giant molecular compounds formed by a large number of atoms that are covalently bonded together to form a highly regular lattice.
- Diamond and graphite are examples of such covalent network structures.
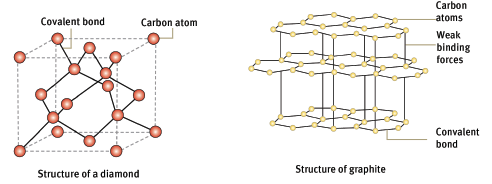
- In diamonds, each carbon atom is attached to four other carbon atoms.
- Graphite consists of layers of carbon atoms. Each carbon atom is attached to three other atoms within the layer. The layers are weakly bonded together and can slide apart easily, which means that graphite is soft. Graphite is used in pencils and as a lubricant in machinery.
6.4 Metallic substances
- A metal substance is one that forms when a group of atoms has a pool of delocalised electrons that surround a lattice of regularly spaced positive ions.
- Most metals are shiny and hard.
6.5 Summary
Bonding: | Ionic: (between metals and non-metals) | Covalent (between non- metals) | Metallic (between metals) | |
Structure: | Giant ionic | Covalent network | Molecular | Giant metallic |
Example: | Sodium chloride
| Diamond
| Iodine
| Zinc
|
Introduction to Physical Science - Physical Science Grade 10 Study Guide
Revision
When revising, many people find it helpful to write as they work. You are more likely to remember something that you have written than something that you have just looked at in a book. You will also find that you can concentrate better and learn faster if you revise hard for a few short sessions rather than for one long one. You will find that you get more revision done in three half-hour sessions with five minute breaks in-between than in one session of 1½ hours. When you take a break, do something completely different – preferably physical. Go for a walk, jump up and down, run around the garden or kick a ball.
Your memory recall of the work you have learned will be improved immensely if you go through it at regular intervals. People who have studied memory talk about the ‘forgetting curve’. Suppose you have done an hour’s revision and have learned a
summary of a topic. The forgetting curve shows that whatever you are going to forget of that summary, you are likely to forget as much as half of it in the next 24 hours. If you spend just five minutes quickly going through that same summary the next day, and another five minutes a few days later, your memory recall at a later date when you write the exam will be much better.
How to tackle exam questions
Multiple choice questions
You probably will have to answer the questions by filling in blocks on an answer sheet. Use a pencil to fill in the blocks, so that you can rub it out if you wish to change an answer. If the examination requires you to use a pen, go over them again at the end when you are satisfied with your answers.
There will usually be four options to choose from in a multiple choice question. When you read the question, try to answer it in your mind without looking at the choices, then see if your answer is one of them. Sometimes the wrong choices can confuse you. There is always only one correct answer, so never fill in two blocks. If you do that, your answer will be marked wrong.
You do not lose marks if you get a multiple choice question wrong, so never leave out a question simply because you are not sure of an answer. Try to eliminate some choices that you think are definitely wrong, and then guess and hope for the best. Do not go on to the next question without committing yourself to an answer to the previous question, even if you are not sure of it. Answer it, but make a mark on the question paper so that, if you have time, you can come back to it when you have finished the rest of the examination.
Calculations
Any answer to a question that requires a calculation must start with a statement of the principle, law or equation that is required for the calculation. If you do not state the formula first and only write down numbers and an answer, you will get no marks, even if your answer is correct.
We use the SI system of units. If you are given a value in another unit, it first must be converted into the relevant SI unit before you substitute it into the equation. It is not necessary to write the unit with each substitution in the equation, provided each is in the correct SI units. You must write the correct SI unit with you final answer.
So the procedure is as follows:
- Ensure that all given quantities are in SI units.
- Write the relevant equation for the calculation. If necessary, change the subject of the formula.
- Substitute the given values. It is not necessary to write the unit with the substitution.
- Carry out the calculation.
- Write the answer, with the correct SI unit. If the quantity is a vector quantity, write the direction.
Mark allocation
Marks are usually allocated as follows:
- One mark for the equation for the calculation.
- One mark for each correct substitution, in SI units.
- One mark for the correctly calculated answer, with the unit. If the unit is missing or incorrect, this mark is lost.
- One mark for the statement of the correct direction, if it is a vector quantity.
Positive marking
Very often questions requiring calculations are structured so that an answer to one part of the question is used in another part of the question. If you make a mistake in the first part so that the answer to that part is wrong, you will not be penalised for an incorrect answer in the later part, provided your calculations are correct. This is often called ‘positive marking’. Nevertheless, what should you do if you have no idea how to answer (say) question 2.1, but know that if only you had the answer to 2.1 you could answer 2.2? Simply assume an answer to 2.1. Write ‘2.2 Assume the answer to 2.1 is …’. Write any number with the correct unit and carry on.
How to use this study guide
Each topic is presented as a summary followed by a selection of examination-type questions. The summaries are the ‘bare bones’ of what you need to know for each topic. Do not try simply to learn the summaries off by heart. You must make sure that you understand each statement in the summary. If not, then refer to the Learner’s Book and study the relevant section. Once you are sure that you understand the statements, you can concentrate on learning the summary. It will be useful for you to write down the key words as they appear in the summary, then test yourself to see if you can state what is in the summary. Then work through the questions set on the topic. The answers are given at the back of the book, with an indication of how marks would be allocated in an exam.
A full sample Physics examination and a full sample Chemistry examination are also provided, with answers for you to test yourself before the final exams at the end of the year.
Prefixes and units
You will encounter the prefixes given in the table below as you study Physical Science. You will see from the table that the prefixes that are used in science all relate to exponents that are multiples of 3. While there are prefixes for numbers bigger than 106 and also smaller than 10-15, it is sufficient for you to learn only those that are in this table.
Prefix | mega- | kilo- | unit | milli- | micro- | nano- | pico- | femto- |
| Factor | × 106 | × 103 | 1 | × 10–3 | × 10–6 | × 10–9 | × 10–12 | × 10–15 |
| Symbol | M | K | m | μ | n | p | f | |
Example | MW º megawatt | kW kilowatt | W Watt | mW milliwatt | μW microwatt | nW nanowatt | pW picowatt | fW femto- watt |
- Micro uses the Greek symbol μ (pronounced ‘mew’).
- All prefix symbols are small letters except for mega. This is to distinguish mega from milli.
- When writing the symbol for the prefix with the symbol for the unit (for instance, mW) there is no space or dot between the prefix and the unit.
SI units used in the Grade 10 curriculum
Here is a list of the symbols and SI units for quantities that you will come across in the Grade 10 curriculum. Test yourself to see that you know the symbol and unit for the quantity and the quantity for the unit.
Temperature | T | K (kelvin) |
Distance | D | m |
Amplitude | A | m |
Frequency | f | Hz (hertz) |
Time | t | s |
Period | T | s |
Speed, velocity | v | m.s–1 |
Wavelength | λ | m |
Energy | E | J (joule) |
Planck’s constant | h | J.s |
Charge | Q | C (coulomb) |
Potential difference | V | V (volt) |
Emf | E | V (volt) |
Current | I | A (ampere) |
Resistance | R | Ω (ohm) |
Quantity of matter | n | mol |
Volume | V | m3 (or dm3 for concentration) |
Concentration | c | mol·dm–3 |
Pressure | p | Pa (pascal) |
Acceleration | a | m.s–2 |
Final Examination Revision Papers - Agricultural Sciences Grade 10 Study Guide
Paper 1
TIME: 2 HOURS
SECTION A
QUESTION 1
1.1 Various options have been given as answers to the following questions. Choose the correct answer and write only the letter (A – D) next to the question number in the ANSWER BOOK.
1.1.1 Hot deserts are regions where evaporation of water is the rainfall.
- lower than
- higher than
- similar to
- double.
1.1.2 Animals living in the grass tundra have adapted to the very cold conditions in a variety of ways. Indicate which of the adaptations below would be the most effective adaptation:
- A thick layer of fat below the skin
- Growth of dark coloured fur / pelts
- Thin pelts to help regulate body temperature
- Eating large volumes of food.
1.1.3 Typical savannah vegetation is
- proteas.
- short grass with shrubs
- tall grass with small deciduous trees.
- indigenous forests.
1.1.4 The practice of keeping/breeding animals under natural conditions in a certain ecological environment, is called production.
- natural livestock
- intensive livestock
- cattle
- extensive livestock
1.1.5 A cattle breed that has a thick hide that makes it difficult for lice or ticks to penetrate the skin is the
- Afrikaner.
- Hereford.
- Drakensberger.
- Jersey.
1.1.6 Reasons for the development of new breeds and plant cultivars:
- Adaptation
- Higher production
- Better quality
- All the above-mentioned
1.1.7 An example of a plant that can survive in dry/arid environmental conditions and which is mostly found growing on northern slopes, is called a/an
- sciophyte.
- xerophyte.
- hidrophyte.
- epihypte.
1.1.8 A dormant stage of animals in winter months is called
- estivation.
- hibernation.
- precipitation.
- astrophication.
(8 x 2) (16)
1.2 Give ONE-word answers for each of the statements or explanations below:
1.2.1 A farmer that grows just enough food for the family to live on
1.2.2 The practice of growing the same crop in the same soil continuously
1.2.3 A plant family that fixes nitrogen in the soil
1.2.4 Returning plant residues to soil to provide nutrients
1.2.5 The cattle breed that adapted to the African continent over the centuries
1.2.6 The practice where different breeds are interbred to develop a new breed
1.2.7 The increase of a country’s population that results in the need for greater food production
1.2.8 The dairy breed that produces the highest butter fat in milk
(8)
1.3 Choose an item/word from COLUMN B that matches the description/item/word in COLUMN A.
COLUMN A | COLUMN B |
1.3.1 Natural resources 1.3.2 Subtropical fruits 1.3.3 Symbiosis |
|
(3 x 2) (6)
1.4 Each of the following sentences consists of TWO statements. Choose the correct statement as follows:
- If the 1st statement is TRUE write A
- If the 2nd statement is TRUE write B
- If both statements are TRUE write C
1.4.1 | Government plays an important role in developing links with other countries | because | it negotiates trade agreements to ensure good markets for businesses. |
1.4.2 | An ant and plant lice are examples of mutualism | because | in mutualism one individual derives benefit and the other not. |
1.4.3 | People can produce food for themselves | because | they are directly dependent on agricultural activities for their food. |
1.4.4 | Lucerne is not capable of binding nitrogen | because | it contains no nitrogen fixing bacteria. |
(5 x 2) (10)
TOTAL SECTION A: 40
SECTION B
QUESTION 2
2.1 Study the distribution of rainfall in South Africa as shown in the map and key that accompanies the map.
2.1.1 Indicate what regions (A to F) would be more likely to have these individual rainfall figures. (Write down the LETTERS with the appropriate rainfall figures in the answer book.) (6)
2.1.2 Identify the region as indicated below.
- Region D
- Region B (2)
2.1.3 In which region would one find:
- Succulent plants
- Subtropical rainforests (2)
2.2
2.2.1 Explain what the difference is between the following:
- Sour veld
- Sweet veld. (2 x 2) (4)
2.2.2 Name the TWO abiotic factors that determine the type of veld found in the different ecological regions of South Africa. (2)
2.3 Read the following paragraph and answer the questions that follow:
The San People
The San are the oldest inhabitants of Southern Africa. They are a group of about 100 000 people who know a lot about the plant and animal life of the Kalahari and have learnt how to survive in this harsh environment. They are hunter-gatherers by tradition and have
excellent animal tracking and hunting skills. Many of them have been forced off their lands to live in settlement areas where they are unable to hunt or gather food.
The San discovered that the Hoodia succulent cactus plant prevented them from getting hungry and thirsty – very useful when on long hunting journeys. Their indigenous knowledge is now being used to produce a drug for dieting. The San people are very poor and would like to be paid by the drug companies that are using their knowledge.
2.3.1 Name THREE systems of farming used in South Africa today. (3)
2.3.2 The San migrated to gather food. What sort of food did they eat traditionally? (2)
2.3.3 Why do you think did the San people lose their habitat? (1)
2.3.4 What Act did the government put in place to redress the imbalances of land ownership of the past? (1)
2.3.5 Give TWO reasons for modern day migration, away from rural areas. (2)
2.3.6 The San’s indigenous knowledge is being put to use by producing a secondary industrial product. Name this product. (2)
2.4 Developing secondary industries is a natural process in meeting the demand of communities. Indicate what raw materials were used to produce the following products:
2.4.1 Wine
2.4.2 School shirts
2.4.3 Bacon
2.4.4 Bread (4)
2.5 During each wet or dry cycle that occurs every seven to nine years, South Africa received an above or below average rainfall just as was experienced in Mozambique a few years ago. These weather phenomena have a devastating effect on agricultural production. Name and give the reasons for each of these weather phenomena:
2.5.1 The dry period in a wet cycle
2.5.2 The above average rainfall or even floods (2 x 2) (4)
[35]
QUESTION 3
3.1 Study the water cycle illustrated below and answer the questions that follow:
3.1.1 What is indicated by the letters A to D. (4)
3.1.2 Indicate ONE method how the water loss as indicated by A to D, can be minimised. (4)
3.2
3.2.1 Why is the building of dams advantageous for agricultural production? Also state TWO negative effects of dams being build for water storage. (2)
3.2.2 What do we call the artificial application of water? (1)
3.3 Give the meaning of the following:
3.3.1 Herbivore
3.3.2 Carnivore (2 x2) (4)
QUESTION 3
4.1 Indicate which of the livestock is best suited to produce the following products:
| Dorper, Jersey, Australop, Dormer, Merino, Drakensberger, Angora,Dorper, Jersey,Australop, Dormer, Merino, Drakensberger, Angora,Large White, Frieslander, Simmentaler, Leghorn, Saannen |
4.1.1 Red meat production
4.1.2 Milk production
4.1.3 Wool production
4.1.4 Egg production
4.1.5 Meat and egg production
4.1.6 Mohair production
4.1.7 Meat and milk production
4.1.8 Meat and wool production
4.1.9 Goats milk production
4.1.10 Pork production (10)
[25]
TOTAL SECTION B: 60
Paper 2
TIME: 11⁄2 HOURS
SECTION A
QUESTION 1
1.1 Various options have been given as answers to the following questions. Choose the correct answer and write only the letter (A – D) next to the question number in the ANSWER BOOK.
1.1.1 The soil forming mineral that is a clear crystal, very hard, transparent to milky white and does not weather is
- biotite.
- quartz.
- augite.
- calcite.
1.1.2 The soil-forming mineral that weathers to form soils rich in phosphates is
- apatite.
- quartz.
- agite.
- calcite.
1.1.3 In the prevention of pollution it is important that the pesticides used for agricultural purposes must
- be biodegradable.
- have after effects.
- not be biodegradable.
- disrupt the food chain.
1.1.4 An example of physical weathering is
- running water.
- wind.
- carbonic acid.
- temperature changes.
1.1.5 The indigenous management of fungal diseases and insects was done by
- using onion and garlic extracts.
- using chemical insecticides.
- using DDT.
- removing weeds between the crops planted.
1.1.6 Which mineral is an example of a toxic ion in irrigation water?
- Boron
- Iron
- Calcium
- Magnesium Agricultural Sciences
1.1.7 An example of the plant family Graminaceae is
- wheat
- beans
- groundnuts
- soya beans
1.1.8 Veld management systems must be based on the principle of
- zero grazing.
- severe grazing.
- rotational grazing.
- selective grazing (8 x 2) (16)
1.2 Give ONE word answers for each of the statements below:
1.2.1 The practice where crops are planted without disturbing the soil
1.2.2 The practice where only one crop is grown over and over on the same land
1.2.3 The practice where plant residues are returned to the soil to provide nutrients
1.2.4 The farming method where a farmer grows just enough food for the family
1.2.5 The farming method where a farmer produces on a large scale for profit
1.2.6 The family that legumes belong to
1.2.7 The process refers to organisms changing organic material into humus
1.2.8 The process when minerals in rocks come into contact with oxygen (8)
1.3 Study the different types of crops in COLUMN B and match them with the information provided in COLUMN A. (6)
COLUMN A | COLUMN B |
1.3.1 Oil seeds | A kikuyu |
1.3.2 Field crops | B tobacco |
1.3.3 Horticultural crops | C deciduous fruits |
D sunflowers |
1.4 Draw a rough map of South Africa. On it, show where the horticultural crops in the list below that grow well.
1.4.1 Wine grapes
1.4.2 Citrus
1.4.3 Bananas
1.4.4 Cherries
1.4.5 Apricots (5)
TOTAL SECTION A: 35
SECTION B
QUESTION 2
2.1 Study the diagram showing the different layers of the earth and answer the questions that follow:
2.1.1 In which layer do we find magma? (1)
2.1.2 Define the term magma. (2)
2.1.3 Briefly describe the economic importance of the crust. (2)
2.1.4 Define igneous rocks and give an example of each and where they are formed. (5)
2.1.5 What do we call the continuous process of solid rock breaking down to form soil particles? (2)
2.2
2.2.1 Describe the term bare cultivation. (3)
2.2.2 Give FIVE disadvantages of this method of cultivation. (5)
2.2.3 What farming practice would you use to prevent erosion on the slopes? Give a reason for your answer. (2)
2.2.4 Explain the process of weathering. (3)
[25]
QUESTION 3
3.1
3.1.1 Describe step by step how you would go about laying out a compost heap. (6)
3.1.2 How would you go about testing the compost heap to see whether decomposition is actively taking place? (2)
3.1.3 Name FIVE benefits that compost will have for your soil. (5)
3. 2 Cultivated pasture plays a very important role in a sustainable supply of food. Briefly discuss this statement. (4)
3.3 Read the stories about the soil utilisation taking place on two different family farms.
The Mbuza family farm
Every member of the family helps on the farm. Lindiwe helps her grandma collect eggs and feed the chickens. She also helps to hoe the soil and dig in chicken manure. Bongani helps to milk the cows and move them from one pasture to another.
Vegetables also grow well. They make sure never to plant the same vegetables in the same soil two years in a row. ‘The vegetables all need different things from the soil,’ they say, ‘so if we do not plant the same things in the same soil, we give the soil a chance to re- build itself.’
The Mbuza pastures have good grass and the animals are fat. The animals are moved to different pastures to prevent the animals from eating all the grass. ‘The soil gets dry and blows away in the wind because there are no roots to keep it in place,’ says Mr Mbuza. Bongani helps his father to make fences between the pastures
The Nkondo family farm
They live near the Mbuza family. Although they have more cows and goats than the Mbuza family, they have not built fences around different pastures, so the animals graze all over the farm all the time.
The family used to grow vegetables but now they only grow mealies. ‘We bought a small tractor with the money that we get from selling our mealies,’ says Mrs Nkondo, ‘but I don’t think it is so good for the soil because it squashes the soil and only breaks it into big lumps.’
Lately the mealie production on the Nkondo farm has not been so good. The soil is dry and blows away in the wind.
3.3.1 Describe TWO methods used by the Mbuza family to keep their pastures and animals growing well. (2 x 2) (4)
3.3.2 List THREE reasons why the Nkondo family’s mealies are not growing well. (3)
3.3.3 Explain why the Nkondo animals are in poor condition. (2)
TOTAL SECTION B: 25
Memorandum: Paper 1
Question 1.1
1.1.1 B
1.1.2 B
1.2.3 D
1.1.4 D
1.1.5 C
1.1.6 A
1.1.7 B
1.1.8 C
Question 1.2
1.2.1 Subsistence
1.2.2 Monoculture
1.2.3 Legume
1.2.4 Zero tillage
1.2.5 Nguni
1.2.6 Crossbreeding
1.2.7 Population growth rate
1.2.8 Jersey
Question 1.3
1.3.1 A
1.3.2 E
1.3.3 F
Question 1.4
1.4.1 C
1.4.2 A
1.4.3 A
1.4.1 B
Question 2.1
2.1.1 A = less than 200 mm; B = 201 – 400 mm; C = 401 – 600 mm; D = 601–800 mm; E = 801– 1 000 mm; F = more than 1 000 mm
2.1.2
- D = Parts North West; Limpopo, Gauteng;
- B = Parts of Northern Cape, Western Cape and Eastern Cape
2.1.3
- A;
- F
Question 2.2
2.2.1
- Sour veld = Sour veld develops in areas with high rainfall more than 650mm; low rainfall with cold winter areas;
- Sweet veld = Sweet veld found in areas with low rainfall areas, 250–500 mm; high temperatures almost no frost
2.2.2 Adaptation of grasses to rainfall and the temperature, will determine the veld types
Question 2.3
2.3.1 Subsistence; small-scale; commercial
2.3.2 Game; certain roots and leaves
2.3.3 They were forced off their land by economic development: mining and agriculture
2.3.4 Restitution Act of 1994
2.3.5 People look for work and for a better life (education, medical care, etc.)
2.3.6 Hoodia succulent cactus plant
Question 2.4
2.4.1 Grapes
2.4.2 Cotton
2.4.3 Pork (pigs)
2.4.4 Wheat
Question 2.5
2.5.1 El Niño – temperatures over equatorial Pacific become warmer = more precipitation
2.5.2 La Niña – temperatures over equatorial Pacific become cooler = less precipitation
Question 3.1
3.1.1 A = Ocean evaporation; B = Land evaporation/transpiration; C = Percolation; D = Runoff
3.1.2 Mulching (prevent evaporation); contour ploughing (minimise runoff)
Question 3.2
3.2.1 Stores water for when it needed; makes water available where needed. Alters river courses; displaces people; causes siltation; can cause flooding
3.2.2 Irrigation
Question 3.3
3.3.1 Plant eating
3.3.2 Meat eating
Question 4.1
4.1.1 Drakensberger
4.1.2 Jersey; Frieslander
4.1.3 Merino
4.1.4 Leghorn
4.1.5 Australop
4.1.6 Angora
4.1.7 Simmentaler
4.1.8 Dormer
4.1.9 Saannen
4.1.10 Large White
Memorandum: Paper 2
Question 1.1
1.1.1 B
1.1.2 C
1.1.3 A
1.1.4 C
1.1.5 A
1.1.6 A
1.1.7 A
1.1.8 D
Question 1.2
1.2.1 Minimum cultivation / no-till
1.2.2 Mono culture / mono cropping
1.2.3 Mulching
1.2.4 Subsistence farming
1.2.5 Cash-crop farming
1.2.6 Leguminaceae
1.2.7 Humification
1.2.8 Oxidation
Question 1.3
1.3.1 D
1.3.2 B
1.3.3 C
Question 1.4
1.4.1 Western Cape
1.4.2 Eastern Cape (and around Citrusdal in Western Cape)
1.4.3 KwaZulu-Natal
1.4.4 Southeastern Free State
1.4.5 Boland
Question 2.1
2.1.1 Inner core
2.1.2 Melted rock / cooled down gas
2.1.3 Soil is formed in the crust where agricultural production takes place
2.1.4 Igneous rocks form due to cooling down of magna Granite – very deep cooling down slowly
Dolorite/ gabbro / basalt – shallow with a fine structure
2.1.5 Weathering
Question 2.2
2.2.1 A practice where all the plants are removed from the soil
2.2.2 Destroys structure and therefore weakens aeration; weakens water infiltration; weakens water capacity; hampers root penetration; difficult to till
2.2.3 Contour ploughing – the contours prevent runoff which prevents soil being washed away
2.2.4 When rocks are subjected to soil forming factors, such as weathering, over a period, the outer layer of the rocks loosens and crumbles to form soils. Rock weathering takes place through mechanical weathering, chemical weathering and biological weathering.
Question 3.1
3.1.1 Base – maize stalks; organic material, e.g. grass and leaves; manure; wood ash; lime; topsoil; dry leaves; water
3.1.2 Wire pushed into centre of compost heap – warm when taken out after 24 hrs / Open slightly – warm centre / Push hand in – warm inside
3.1.3 Improved structure; improved aeration; improved inflitration; improved water capacity; easier tillage; better root penetration
Question 3.2
Dung of cattle will fertilize the soil and:
- the urine will provide moisture and helps to form humus.
- After removing the cattle, the dung must be dug into the soil immediately to provide organic matter.
Question 3.3
3.3.1 Rotational grazing – camps rest to recover; Crop rotation – soil minerals replenished
3.3.2 Overgrazing – no rotation of grazing in camps; Over tiling – soil structure broken down; Mono crop cultivation – soil not being replenished
3.3.3 Over populated – carrying capacity of veld exceeded (should lessen animal numbers and practise rotational grazing)
Agricultural Science Grade 10 Glossary - Agricultural Science Grade 10 Study Guide
GLOSARRY
A
- abiotic physical rather than biological; not derived from living organisms
- abrasion the process by which clasts and other particles are reduced in size
- adaptation a change by which an organism or species becomes better suited to its environment
- aeration the introduction of air into a material or substance
- agents of erosion water, wind and glaciers
- agricultural policies a course or principle of action related to agriculture that is adopted or proposed by the government
- agricultural production the output from agriculture
- agro-ecology the study of the relationship between the environment and agricultural plants and animals
- agronomy the science of soil management and crop production
- alluvial deposits when loose alluvial material is deposited or cemented into stone (lithified)
- alluvium loose, unconsolidated (not cemented together into a solid rock), soil or sediments, eroded, deposited, and reshaped by water in some form in a non-marine setting. Alluvium is typically made up of a variety of materials, including fine particles of silt and clay and larger particles of sand and gravel
- amendment a change or addition to a legal or statutory (to do with the laws of a country) document
- anaerobic absence of free oxygen
- animal ratio the ratio of different animals, or of male to female animals in a pasture
- annual population growth rate the rate at which the population increases or decreases in a given year, expressed as a percentage of the total population
- artificial pasture an area with plants planted specifically for grazing
- average annual rainfall the amount of rain (measured in millimetres or mm) that usually falls in an area in one year
B
- biodegradable when something decomposes naturally
- biodiversity the variety of life in the world or in a particular habitat or ecosystem
- biological farming systems farmers use biological principles to increase their yields
- biological matter living matter
- biological pest control farmers use a pest’s natural enemy to get rid of it
- biological weathering when living organisms cause rocks to decompose
- biome a large naturally occurring community of flora and fauna occupying a major habitat
- biosphere the part of the Earth that supports life, including oceans, lakes and rivers
- biotic of, relating to, or resulting from living things, esp. in their ecological relations
- bioturbation the disturbance of sedimentary deposits by living organisms
- bivalent homologous chromosomes associated in pairs
- breed specific variety of a species
C
- capillary action the ability of liquid to flow against gravity, such as up a plant root
- carbohydrates one of the five nutrients in food; used by the bodies of people and animals to get energy
- carbon dioxide (CO2) a colourless, odourless gas produced by burning carbon and organic compounds and by respiration; it is naturally present in air (about 0,03%) and is absorbed by plants in photosynthesis
- carcass traits meat characteristics after slaughter
- carrying capacity the number of animals that can be grazed on a piece of land
- cell cycle the sequence of cell division, resting phase, cell division and so on
- cell membrane the ‘skin’ of a cell, also known as the plasmalemma or plasma membrane
- cell wall a non-living tissue made up from cellulose that is situated outside the cell membrane of a plant cell
- centromere the point on a chromosome by which it is attached to a spindle fibre during cell division
- chemical (or biological) weathering involves the direct effect of atmospheric chemicals or biologically produced chemicals in the breakdown of rocks, soils and minerals
- chromatid each of the two threadlike strands into which a chromosome divides longitudinally during cell division. Each contains a double helix of DNA
- chromatin network a network of fine threads consisting of protein, RNA and DNA
- chromosome a threadlike structure of nucleic acids and protein found in the nucleus of most living cells, carrying genetic information in the form of genes
- cleavage the splitting of rocks or crystals in a preferred plane or direction
- climate change long-term, significant change in the climate of an area or of the Earth, usually seen as resulting from human activity
- climate the weather conditions prevailing in an area in general or over a long period
- climax species plant species that will remain essentially unchanged in terms of species composition for as long as a site remains undisturbed
- colluvium loose bodies of sediment that have been deposited or built up at the bottom of a low-grade slope or against a barrier on that slope, transported by gravity
- commensalism one organism benefits from the relationship, while the other organism is not harmed
- commercial farming sector large-scale farming for profit
- commercial production scientific farming on a large scale for profit
- communal farming when animals are allowed to roam freely across the farm, without any fences and grazing camps
- community all the plants and animals that live in a particular ecosystem
- competition when organisms within the same ecosystem want the same resources, for example food
- competitive edge a unique selling point; something special that can be used to sell a product
- compost decomposed plant matter that is added to soil to increase the nutrient content of the soil; organic matter that has broken down into humus
- condensation when a vapour changes into a liquid
- conformation body structure and shape
- conservation farming use of natural ecological processes to manage farms
- conservation protecting a species
- conserve to save and to use wisely
- constitution a body of fundamental principles or established precedents according to which a state or other organisation is acknowledged to be governed (in South Africa, the Constitution of 1996 is the highest law in the land, and all other laws are only legal if they are allowed by the Constitution)
- consumer price index (CPI) measures the average changes over time of prices paid for goods and services
- contaminants substances that decrease the quality of something and make it unhealthy
- continuous grazing when animals graze the same area at all times of the year
- contour a line on a map joining points of equal height above or below sea level; ploughing along the contours of the land in order to minimise soil erosion
- conventional farming methods methods to increase yields by using artificial additives, without considering damage to the environment
- crop rotation growing a different crop on the same land each year for a period of three to five years
- crossbreeding mating of two different breeds
- cultivar a plant variety that has been produced in cultivation by selective breeding
- cytokinesis the cytoplasmic division of a cell at the end of mitosis or meiosis, bringing about the separation into two daughter cells
- cytoplasm the jelly-like fluid inside a cell, excluding the nucleus, in which all the organelles are suspended
D
- deciduous a tree or shrub that sheds its leaves annually
- decomposers organisms that break down dead plants and animals through a process called decay
- deforestation the cutting down of large areas of forests
- denitrification removal of nitrates or nitrites from soil, air or water
- diluents a substance used to dilute another liquid (make it thinner )
- diploid containing two complete sets of chromosomes, one from each parent.
- DNA the abbreviation for deoxyribonucleic acid, which is the carrier of genetic information
- drought-tolerant plants plants that can grow with less water, and continue to grow if little rain falls
- dual agricultural economy two separate economies, commercial and communal
- dyke an intrusion of igneous rock cutting across existing strata
E
- ecological farming systems using ecological and natural principles to increase yields
- ecological pyramid a diagram to show the trophic levels in an ecosystem
- ecological succession when the type of plants growing in a pasture changes naturally without the interference of people
- ecology the study of the relationships between living organisms and the environment in which they live
- economy the wealth and resources of a country or region, especially in terms of the production and consumption of goods and services
- ecosystem a biological community of interacting organisms and their physical environment
- edaphic of, produced by, or influenced by the soil
- El Niño weather phenomenon as a result of the ocean off the South American coast becoming warmer than normal
- eluviation the transport of soil material from upper layers of soil to lower levels by downward precipitation of water across soil horizons; this transported material is called illuvial deposit
- endangered species a species in danger of disappearing
- endoplasmic reticulum a network of membranous tubules (very small tubes) within the cytoplasm of a cell
- enzymes substances in living organisms that control chemical processes, for example digestion
- equine family members of the family Equidae which includes horses, donkeys and zebras
- erode when wind, water or other natural agents gradually wear away soil, rock or land
- erosion the gradual removal of soil by wind or water; it is a natural process but can be increased by poor farming practices and deforestation
- evaporation when a liquid changes into a gas
- exotic breed breed developed in another country with very different conditions
- exotic come from some other parts of the world; not indigenous
F
- fauna the animals of a particular region or habitat
- feed (or food) conversion efficiency how well the animal converts protein in feed into growth
- fertility how fruitful or productive something is
- fibre diameter width of fibre
- fibre length how long the fibre is
- flora the plants of a particular region or habitat
- floral kingdom the largest natural units determined for flowering plants
- fodder food, especially dried hay or feed, for cattle and other livestock
- follicle density the number of follicles per area of skin
- food chain a hierarchical series of organisms each dependent on the next as a source of food
- food security the availability of enough nutritious food and people’s access to it
- food web a system of interlocking and interdependent food chains
- forage plant material eaten by grazing livestock
- foreign currency money a country earns from outside the country
- fossil fuels fuels made from coal, which is plant material that was compacted and fossilised many years ago
- fracture the physical appearance of a freshly broken rock or mineral, especially as regards the shape of the surface formed
- friable (soil) easily crumbled (soil)
G
- game an old English word for wild animals used for food; now refers to wild animals such as buck
- gametes a mature haploid male or female germ cell that is able to unite with another of the opposite sex in sexual reproduction to form a zygote
- geology geology of an area refers to the origin and evolution of the materials the soil consists of, and the processes that act on it
- germination when a seed begins to grow and put out shoots after a period of dormancy
- global demand to do with how many goods or services are needed or wanted internationally
- global warming the phenomenon of the temperature of the Earth increasing due to the greenhouse effect, mainly caused by pollution
- globalisation the process by which regional economies, societies, and cultures have become integrated through communication, transportation, and trade
- Golgi apparatus folded membranes within the cytoplasm of most cells, involved in secretion and intracellular transport
- grazing capacity the average number of animals that the farm can sustain over a period of time
- grazing ecology the study of the relationship between the organisms in the ecosystem of the pasture
- grazing system a particular grazing strategy that a farmer uses
- green manure a plant that is sown to add nutrients and organic matter to the soil; it is ploughed into the land after a period of time
- green paper also known as consultation documents, proposes a strategy to be implemented or sets out proposals on which the government wishes to obtain public views and opinion
- gross domestic product (GDP) the total value of goods produced and services provided in a country during one year
- gross value total amount earned for all products in one year
- groundwater water that collects under the ground above a layer of rock and in cracks in the rocks
- growth rate the speed of animals’ growth
H
- haploid having a single set of unpaired chromosomes
- hardwood broad-leaved deciduous trees, such as oak, ash or beech
- herbaceous is a plant that has leaves and stems that die down at the end of the growing season to the soil level; they have no persistent woody stem above ground; they may be annuals, biennials or perennials
- herbicides chemicals that kill plants; farmers use herbicides to control weeds
- homologous pairs pairing at meiosis and having the same structural features and pattern of genes
- horticulture the art or practice of garden cultivation and management
- humid marked by a relatively high level of water vapour in the atmosphere
- humification converting plant remains into humus
- humus any organic matter that has reached a point of stability, where it will break down no further and might, if conditions do not change, remain as it is for centuries, if not millennia; the organic component of soil, formed by the decomposition of leaves and other plant material by soil microorganisms
I
- illuviation the accumulation of illuvial deposit in lower levels
- indigenous breed breed developed locally
- indigenous knowledge knowledge that has been handed down in communities, over thousands of years by word of mouth
- individuals members of a population
- industrialisation a period characterised by an increase in mechanisation and factories
- inorganic substances that do not contain carbon
- invader plants plants of alien or foreign origin; weeds
- invasive unwanted, spreading quickly in an uncontrolled way that can cause harm
- irrigation supplying water to a crop by moving water from the source of the water to the place where the crop is growing
L
- La Niña opposite of El Niño; weather phenomenon as a result of the ocean off the South American coast becoming cooler than normal
- land reform changing land laws to make the situation fairer for all people; redressing previous inequalities in land distribution
- land restitution actions to reverse injustices related to land ownership
- leaching making a soluble chemical or mineral drain away from the soil by the action of a percolating liquid such as rainwater
- leasehold the holding of property by lease, not ownership
- legal framework basic structure underlying the system of laws; such a framework creates the legal ‘space’ in which individuals and businesses can function
- legislation the laws of a country, as determined by the law-making arm of government, such as parliament
- legume an edible plant that grows inside a pod
- livestock farm animals regarded as an asset
- loamy soil soil composed of sand, silt, and clay in relatively even concentration; loamy soils generally contain more nutrients and humus than sandy soils, have better infiltration and drainage than silty soils, and are easier to till than clay soils; they are gritty, moist, and retain water easily
- lustre the manner in which the surface of a mineral reflects light
- lysosome an organelle in the cytoplasm of cells containing enzymes that break down or degrade substances enclosed in a membrane
M
- management systems how a farm is organised and run
- market requirement what the consumer wants; standards set by the market
- marketing a formal structured approach to selling based on analysis of the subject
- mechanical weathering involves the breakdown of rocks and soils through direct contact with atmospheric conditions, such as heat, water, ice and pressure
- mechanical weed control removing weeds using implements such as hoes
- microbe a microorganism, especially bacterium causing fermentation
- microbial related to microorganisms, such as bacteria, that cause fermentation
- milk production the amount of milk produced by a dairy animal
- minerals inorganic substances needed by the human body for good health
- mitochondrion an organelle found in large numbers in most cells, in which the biochemical processes of respiration and energy production occur; mitochondrion has a double membrane, the inner layer being folded inward to form layers (cristae)
- monogastric animals with a single stomach
- mulching a material (such as decaying leaves, bark, or compost) spread over the soil to enrich or insulate the soil
- multicellular made up of more than one cell
- multiplier effect the variety of products that can be derived from a single raw material
- mutualism two organisms benefit from a relationship
N
- natural pasture an area where animals graze that does not contain any plants planted by the farmer
- natural resources resources that occur naturally in the environment; also called primary resources
- natural selection selection of an animal by the environment for survival
- no-tillage farming planting a crop without ploughing or digging the soil first; the crop is planted into the leaves and stems that are left over from the previous crop
- non-renewable resources natural resources that are limited and may be used up one day
- non-selective grazing when animals are forced to eat all the plants in the pasture, not only the palatable plants
- nucleoplasm the jelly-like fluid in the nucleus
- nucleus dense organelle present in most cells, typically a single rounded structure bounded by a double membrane, containing the genetic material
- nutrients substances in food that animals and people need in order to grow and keep healthy; the minerals in soil are sometimes called plant nutrients
- nutritive value amount of nutrients a plant contains
O
- orchard a piece of land planted with fruit trees
- organ a group of tissues that have the same structure and function
- organelles various structures in a cell, each performing a different function
- organic farming increasing yields by using natural additives, such as compost
- organic matter the remains of plants and animals that use the soil
- organic substances containing carbon
- outwash is a glacial outwash plain formed of sediments deposited by meltwater at the terminus of a glacier
- over-grazing if more animals than the quantity indicated by the stocking rate are allowed to graze the pasture
- oxidation the combination of a substance with oxygen
P
- palatable plants that animals like to eat because they are soft and tasty
- parasitism one organism (parasite) lives on another organism (host), and the host is harmed
- parenchyma the functional tissue of an organ as distinguished from the connective and supporting tissue
- pedogenesis the process by which soil is formed
- pesticides chemical poisons used to control pests and diseases
- pH a figure expressing the acidity or alkalinity of a solution on a logarithmic scale on which 7 is neutral, lower values are more acid, and higher values more alkaline
- photosynthesise the process by which green plants and some other organisms use sunlight to synthesise foods from carbon dioxide and water. Photosynthesis in plants generally involves the green pigment chlorophyll and generates oxygen as a by-product
- physiographic refers to what the land surface looks like, or the appearance of the land surface
- plant taxonomy the science that finds, describes, classifies, identifies and names plants
- plantations an area in which trees have been planted for commercial purposes
- plasmodesmata threads made of cytoplasm that go through from one plant cell to the next
- plastid any of a class of small organelles, such as chloroplasts, in the cytoplasm of plant cells, containing pigment or food
- pollutants harmful substances that pollute the air, water or soil
- pollute to put harmful substances into the air, water or soil
- population growth the rate at which the number of individuals in a population increases
- population the number of organisms (people or animals)
- pores openings in a cell membrane
- precipitation water that comes from the air; it includes rain, hail, frost, snow, dew and mist (fog)
- predation when one organism (hunter) eats another organism (prey)
- primary agriculture the first level of agriculture involved in the planting and cultivation of crops
- primary, secondary and tertiary industries primary industries are concerned with obtaining or providing natural raw materials for conversion into commodities and products for the consumer (mining, agriculture or forestry); secondary industries convert raw materials provided by primary industry into commodities and products for the consumer (also known as manufacturing); tertiary industries are involved in the provision of goods and services
- producers green plants that can make their own food through a process called photosynthesis
- production systems farming methods (for example beef production systems are classified according to the age at which animals emanating from a production unit are sold. The production unit could be a farm or one of the enterprises in a larger undertaking. A full description of a system includes the age, mass and carcass class at which animals are marketed, as well as the breeding, management and feeding practices followed. In South Africa the most common beef production systems are weaner-, long yearling (tolly)- and two-year old (ox) systems.)
- protein an organic compound that is an essential part of all living organisms
- purebred bred from parents of the same breed or variety
R
- radiation sending out rays, for example from the sun
- reafforestation to cover an area by planting trees
- regenerate to change back to the original, new condition
- regulation a rule or directive made and maintained by an authority; legislation is usually implemented through regulations
- renewable resources natural resources that can never be used up over time
- reservoir any place where water is stored
- resource conservation looking after natural resources (e.g. soil and water) to ensure their quality and that they are not used up or wasted (can also refer to looking after other resources, such as money and farm equipment) wisely so that they are of maximum benefit and last a long time)
- ribosomes a minute (very small) particle consisting of RNA and associated proteins, found in large numbers in the cytoplasm of living cells
- RNA abbreviation for ribonucleic acid, a nucleic acid present in all living cells; its main function is to act as a messenger carrying instructions from DNA
- rock weathering the breaking down of Earth’s rocks, soils and minerals through direct contact with the planet’s atmosphere; it includes physical, biological and chemical weathering
- rough ER endoplasmic reticulum that contains ribosomes on its surface
- ruminant hoofed mammals that have a stomach with four compartments; partially digested food is brought back for the animal to chew again, for example cows
S
- salinisation to increase the salt content
- salinity a measure of the total amount of dissolved salts in water or soil
- scientific or evidence-based knowledge knowledge that comes from experimentation and research, and is based on verifiable evidence
- season of use the part of the year when animals can graze the plants
- seasonal distribution how something (e.g. rainfall) is spread out across the seasons
- sediment particulate matter that is carried by water or wind and deposited on the surface of the land or the bottom of a body of water, and may in time become consolidated into rock
- selective breeding selecting specific animals for mating
- selective grazing when animals do not graze all the plants in a pasture, but prefer certain plants
- self-propagation able to increase itself in number or amount
- silage grass or other green fodder compacted and stored in airtight conditions, typically in a silo, without first being dried, and used as animal feed in the winter or in periods where grazing is scarce
- siltation when dams and rivers fill up with eroded soil
- slow rotational grazing when the grazing area is divided into camps, one of which is grazed while the others are rested
- small-scale farmers agricultural production for own consumption with a small surplus for trade
- smooth ER endoplasmic reticulum that does not contain ribosomes on its surface
- sodicity a measure of the amount of sodium-containing salts in water; it is measured using the sodium absorption ratio (SAR)
- softwood conifers, such as pine, fir or spruce
- soil compaction when the weight of heavy machinery compresses the soil, causing it to lose pore space; soil compaction may also occur due to a lack of water in the soil
- soil conservation the process where we stop the degradation of soil and repair the damage so that the condition of the soil improves and is maintained
- soil degradation any process that causes the condition of the soil to lose nutrients (degrade)
- soil erosion the carrying away of soil by wind or water
- specialisation selection for a specific purpose
- species a group of living organisms consisting of similar individuals capable of exchanging genes or interbreeding
- stocking rate (SR) the number of animals that can graze on one hectare of land, for the grazeable part of one year, without damaging the condition of the pasture
- strategic position relating to how an industry is set up and functioning in order to identify long-term or overall aims and interests and the means of achieving them
- streak a long, thin line or mark of a different substance or colour from its surroundings
- sublimation when a solid changes into a gas
- subsistence agriculture agricultural production at a level sufficient only for one’s own use or consumption, without any surplus for trade
- subsistence farming when a farmer only produces enough for the use of his or her own household
- subsoil the deeper parts of soil, beneath the topsoil
- supply chain the chain of the product from the farm to the consumer
- surface water water that is on the surface of the Earth, such as the water in rivers and lakes
- sustainable able to be maintained at a certain rate or level; conserving an ecological balance by avoiding depletion of natural resources
T
- terraces steps that are cut into the slope
- tissue a group of cells that have the same structure and function
- titlehold the ownership of property by title deed, a legal document
- tonoplast a membrane that bounds the chief vacuole of a plant cell
- topography the arrangement of the natural and artificial physical features of an area
- topsoil the top layer of the soil, which is usually more fertile than the lower layers of soil
- transpiration give off water vapour through the stomata (the very small pores on the surface of a leaf)
- trifoliate a plant that has three leaflets
- trophic level each step in the food chain
- turbidity muddiness of irrigation water caused by soil particles and organic materials that are suspended in the water
U
- unicellular made up of one cell
- unpalatable plants that animals do not like to eat, because they are tough or have a bad taste or smell
W
- water cycle the way in which water molecules are cycled from one reservoir to the next
- water holding capacity ability of soil to hold water
- water quality fitness of water for a particular use or for the maintenance of the health of aquatic ecosystems
- watercourse a river, stream, or artificially constructed water channel
- white paper a means of presenting government policy preferences prior to the introduction of legislation; as such, the publication of a white paper serves to test the climate of public opinion regarding a controversial policy issue and enables the government to gauge its probable impact
- wind farm a collection of wind turbines which are used to generate electrical power through their mechanical motions as they are pushed by the wind; the energy generated by a wind farm can be fed directly into the general energy grid after passing through transformers
Z
- zero grazing when animals are not allowed to graze outside, but are kept indoors; fodder is brought to them
- zygote the product of fertilisation of an egg cell by a sperm cell
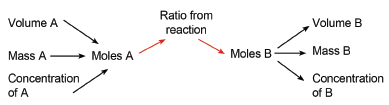
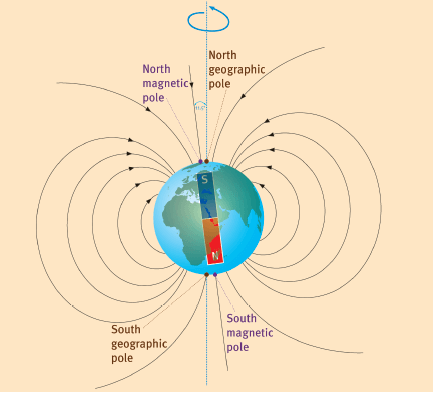
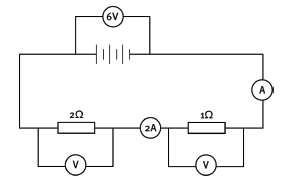
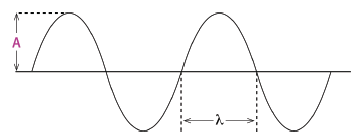
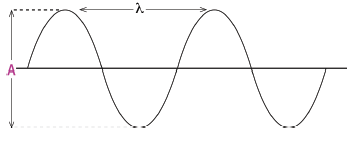
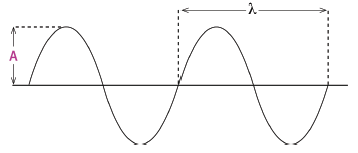
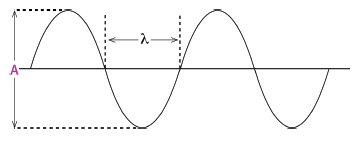 (3)
(3)