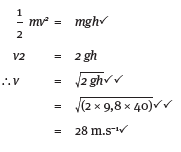Adele
English First Additional Language Paper 3 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
- This question paper consists of THREE sections:
SECTION A: Essay (50)
SECTION B: Longer Transactional Text (30)
SECTION C: Shorter Transactional Text (20) - Answer ONE question from EACH section.
- Write in the language in which you are being assessed.
- Start EACH section on a NEW page.
- You must plan (e.g. using a mind map/diagram/flow chart/key words etc.), edit and proofread your work. The plan must appear BEFORE each text.
- All planning must be clearly indicated as such. It is advisable to draw a line through all planning.
- You are strongly advised to spend approximately:
- 80 minutes on SECTION A
- 40 minutes on SECTION B
- 30 minutes on SECTION C
- Number the answers correctly according to the numbering system used in this question paper.
- Give each response a suitable title/heading.
- Do NOT consider the title/heading when doing a word count.
- Write neatly and legibly.
QUESTIONS
SECTION A: ESSAY
QUESTION 1
- Write an essay of between 250 and 300 words in length on ONE of the following topics.
- Write down the NUMBER and TITLE of the essay you have chosen correctly, for example 1.1. My pleasant/frightening experience
- Give your OWN title if your choice is QUESTION 1.7.1 OR 1.7.2.
- Spend approximately 80 minutes on this section.
1.1 My pleasant/frightening experience [50]
1.2 And now this … [50]
1.3 Changing your mindset [50]
1.4 Choices [50]
1.5 ‘I have decided to stick with love. Hate is too great a burden to bear.’
– Martin Luther King Jnr.
[50]
1.6 Toxic (poisonous) friendship [50]
1.7 Choose ONE of the following pictures and write an essay on a topic that comes to mind. Write the question number (1.7.1 OR 1.7.2) and give your essay a suitable title.
NOTE: There must be a clear link between your essay and the picture you have chosen.
1.7.1
[Source: googlepictures.com] [50]
1.7.2
[Source: googlepictures.com] [50]
TOTAL SECTION A: 50
SECTION B: LONGER TRANSACTIONAL TEXT
QUESTION 2
- Respond to ONE of the following longer transactional writing texts.
- The body of your response should be between 120 and 150 words in length.
- Write down the NUMBER and the HEADING of the text you have chosen, for example
2.1 FRIENDLY LETTER.
- Pay particular attention to format, language and register.
- Spend approximately 40 minutes on this section.
2.1 FRIENDLY LETTER
Your best friend has been the subject of gossip due to an embarrassing experience he/she is alleged to have been involved in. You are concerned that your friend is facing a very difficult time.
Write a letter to your friend in which you comfort him/her and give him/her assurance that the situation is going to change. [30]
2.2 OBITUARY
A famous activist from your community has passed away after a short illness.
Write an obituary in which you highlight the contribution he/she has made to the development of your community. Also pay tribute to him/her. [30]
2.3 INTERVIEW
You applied for a bursary to further your studies in 2021. The company that intends to offer you a bursary has invited you to an interview to find out about your life goals.
Write out the interview between yourself and the director of the company.
NOTE: Use the dialogue format. [30]
2.4 NEWSPAPER ARTICLE
Teenagers often decide to commit suicide when faced with crises in their lives.
Recently a classmate has attempted suicide because of a critical situation he/she found himself/herself in.
Write an article for your school newspaper in which you provide information on how potential suicide victims can be assisted in order not to commit suicide. Also provide information on where to seek help from. [30]
TOTAL SECTION B: 30
SECTION C: SHORTER TRANSACTIONAL TEXT
QUESTION 3
- Choose ONE of the following topics and write a short transactional text.
- The body of your response should be between 80 and 100 words in length.
- Write down the NUMBER and the HEADING of the text you have chosen, for example
3.1 INVITATION CARD.
- Spend approximately 30 minutes on this section.
3.1 INVITATION CARD
The matric class of 2020 at your school wants to honour a favourite teacher. He/She is about to retire after 40 years of service. You have organised a surprise event and you have been asked to design an invitation for this occasion.
Write out the invitation.
NOTE: No marks are awarded for drawings and illustrations. [20]
3.2 DIARY ENTRIES
Recently you have been sick and you were required by your local clinic to have some medical tests done. You are very anxious and worried about these tests as they might affect your schooling.
Write TWO diary entries in which you express how you felt BEFORE you went to have the medical tests done and how you felt AFTER receiving the results of the medical tests. [20]
3.3 INSTRUCTIONS
Your parents had to leave suddenly to care for your grandmother who has become severely sick. You are at home alone and have been asked to write instructions for your domestic worker to do the various tasks at your home.
Write out the instructions. [20]
TOTAL SECTION C: 20
GRAND TOTAL: 100
English First Additional Language Paper 2 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
Read this page carefully before you begin to answer the questions.
- Do NOT attempt to read the entire question paper. Consult the TABLE OF CONTENTS on the next page and mark the numbers of the questions set on texts you have studied this year. Read these questions carefully and answer as per the instructions.
- This question paper consists of FOUR sections:
SECTION A: Novel (35)
SECTION B: Drama (35)
SECTION C: Short Stories (35)
SECTION D: Poetry (35) - Answer TWO QUESTIONS in all, ONE question each from ANY TWO sections.
SECTION A: NOVEL
Answer the question set on the novel you have studied.
SECTION B: DRAMA
Answer the question set on the drama you have studied.
SECTION C: SHORT STORIES
Answer the questions set on BOTH short stories.
SECTION D: POETRY
Answer the questions set on BOTH poems.
Use the checklist on page 4 to assist you. - Follow the instructions at the beginning of each section carefully.
- Number the answers correctly according to the numbering system used in this question paper.
- Start EACH section on a NEW page.
- Suggested time management: Spend approximately 60 minutes on each section.
- Write neatly and legibly.
TABLE OF CONTENTS
SECTION A: NOVEL
Answer ANY ONE question on the novel you have studied.
QUESTION NUMBERS | MARKS | PAGE | ||||||
1. | Cry, the Beloved Country | 35 | 5 | |||||
2. | Strange Case of Dr Jekyll and Mr Hyde | 35 | 9 | |||||
SECTION B: DRAMA | ||||||||
Answer ANY ONE question on the drama you have studied. | ||||||||
3. | Macbeth | 35 | 13 | |||||
4. | My Children! My Africa! | 35 | 18 | |||||
SECTION C: SHORT STORIES | ||||||||
Answer the questions set on BOTH short stories. | ||||||||
5.1 | ‘A chip of glass ruby’ | 17 | 22 | |||||
AND | ||||||||
5.2 | ‘Village people’ | 18 | 24 | |||||
SECTION D: POETRY | ||||||||
Answer the questions set on BOTH poems. | ||||||||
6.1 | ‘Captive’ | 18 | 26 | |||||
AND | ||||||||
6.2 | ‘Mid-term break’ | 17 | 28 | |||||
CHECKLIST
NOTE:
- Answer questions from ANY TWO sections.
- Tick (?) the sections you have answered.
SECTION | QUESTION NUMBERS | NO. OF QUESTIONS TO ANSWER | TICK | |
A: | Novel | 1–2 | 1 | |
B: | Drama | 3–4 | 1 | |
C: | Short Stories | 5 | 1 | |
D: | Poetry | 6 | 1 | |
NOTE: Ensure that you have answered questions on TWO sections only.
QUESTIONS
SECTION A: NOVEL
In this section, questions are set on the following novels:
- CRY, THE BELOVED COUNTRY by Alan Paton
- STRANGE CASE OF DR JEKYLL AND MR HYDE by Robert Louis Stevenson
Answer ALL the questions on the novel that you have studied.
QUESTION 1: CRY, THE BELOVED COUNTRY
Read the extracts from the novel below and answer the questions set on each. The number of marks allocated to each question serves as a guide to the expected length of your answer.
NOTE: Answer the questions set on BOTH extracts, i.e. QUESTION 1.1 AND QUESTION 1.2.
1.1 EXTRACT A
[The search for Absalom begins.]
|
[Book 1, Chapter 8]
1.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (1.1.1(a)–1.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
|
(4 x 1) (4)
1.1.2 Refer to line 1 (‘We shall not use the bus’). Explain why should Kumalo and Msimangu not take the bus. (2)
1.1.3 Refer to line 3 (‘That man has a silver tongue’).
- Identify the figure of speech in this line. (1)
- Explain why this figure of speech is appropriate. (2)
1.1.4 State the difference between John and Dubula in your own words. (2)
1.1.5 Refer to line 15 (‘I am willing to walk’). What does this line tell you about the character of Kumalo? Substantiate your answer. (2)
1.1.6 Refer to lines 17–18 (‘This Johannesburg – it … to be alone’). Discuss the irony in Kumalo’s words in this line. (2)
1.1.7 Absalom is a victim of urbanisation. Discuss your view. (3)
AND
1.2 EXTRACT B
[Kumalo receives a warm welcome in Ndotsheni.]
[Book 3, Chapter 1] |
1.2.1 State TWO reasons why the people of Ndotsheni do not understand the umfundisi they have when Kumalo is away. (2)
1.2.2 Refer to lines 1–2 (‘We did not … he is back’). Explain why it is so important to Kumalo to hear such welcoming words from Ndotsheni people. (1)
1.2.3 Explain in TWO points how Ndotsheni is a wasted land. (2)
1.2.4 Refer to line 12 (‘It is dry … cry for rain’).
- What tone would Kumalo’s friend use in this line? (1)
- Why would Kumalo’s friend use this tone in this line? (1)
1.2.5 Choose the correct answer to complete the following sentence. Write only the letter (A–D) next to the question number (1.2.5) in the ANSWER BOOK.
In the Zulu language ‘Tixo’ in line 14 means …
- Big One.
- Wise One.
- Great Spirit.
- Saviour. (1)
1.2.6 Why is the following statement FALSE? The girl that Kumalo comes back with is Gertrude’s daughter. (1)
1.2.7 Explain why Kumalo comes to Ndotsheni with this girl. (2)
1.2.8 One of the themes evident in the novel is comfort in desolation. Discuss how this theme is relevant to Kumalo and the people of Ndotsheni. (3)
1.2.9 Refer to the novel as a whole. Tragedy brings the two fathers – Jarvis and Kumalo – closer to their sons. Discuss your view. (3) [35]
QUESTION 2: STRANGE CASE OF DR JEKYLL AND MR HYDE
Read the following extracts from the novel and answer the questions set on each. The number of marks allocated to each question serves as a guide to the expected length of your answer.
Answer the questions set on BOTH extracts, i.e. QUESTION 2.1 and QUESTION 2.2.
2.1 EXTRACT C
[Mr Utterson finally meets Mr Hyde.]
Mr Utterson stepped out and touched him on the shoulder as he passed. ‘Mr Hyde, I think?’ [Search for Mr Hyde] |
2.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (2.1.1(a)–2.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
|
(4 x 1) (4)
2.1.2 Refer to lines 1–2 (‘Mr Utterson stepped … Hyde, I think?).
- At what place do Mr Hyde and Mr Utterson meet? (1)
- Explain why the two people meet there. (2)
2.1.3 Refer to line 3 (‘Hyde shrank back … of the breath’).
- Choose the correct answer to complete the following sentence. Write only the letter (A–D) next to the question number (2.1.3) in the ANSWER BOOK.
When Mr Hyde ‘shrank back’ it means that he …- advances towards Utterson.
- approaches Utterson.
- challenges Utterson.
- withdraws from Utterson. (1)
- Explain the relevance of the word ‘hissing’ in the context of this extract. (2)
2.1.4 Refer to line 8 (‘meeting you so … might admit me’). Explain why Mr Utterson says they are meeting ‘conveniently’. (1)
2.1.5 Refer to line 14 (‘Will you let … asked the lawyer’).
- What tone would Mr Utterson use in this line? (1)
- Why would Utterson use this tone in this line? (1)
2.1.6 Describe the atmosphere between Mr Utterson and Mr Hyde in this extract. Substantiate your answer. (2)
2.1.7 Mr Utterson is a good lawyer. Discuss your view. (3)
AND
2.2 EXTRACT D
[Dr Jekyll struggles to keep Hyde under control.]
I made this choice perhaps with some unconscious reservation, for I neither gave up the house in Soho, nor destroyed the clothes of Edward Hyde, which still lay ready in my cabinet. For two months, however, I was true to my determination; for two months I led a life of such severity as I had never before attained to, and enjoyed theI made this choice perhaps with some unconscious reservation, for I neither gave up the house in Soho, nor destroyed the clothes of Edward Hyde, which still lay ready in my cabinet. For two months, however, I was true to my determination; for two months I led a life of such severity as I had never before attained to, and enjoyed the compensations of an approving conscience. But time began at last to obliterate the freshness of my alarm; the praises of conscience began to grow into a thing of course; I began to be tortured with throes and longings, as of Hyde struggling after freedom; and at last, in an hour of moral weakness, I once again compounded and swallowed the transformation draught. I do not suppose that, when a drunkard reasons with himself upon his vice, he is once out of five hundred times affected by the dangers that he runs through his brutish, physical insensibility; neither had I, long as I had considered my position, made enough allowance for the complete moral insensibility and insensate readiness to evil, which were the leading characters of Edward Hyde. Yet it was by these that I was punished. My devil had been long caged, he came out roaring. I was conscious, even when I took the draught, of a more unbridled, a more furious propensity to ill. [Henry Jekyll’s Full Statement of the Case] |
2.2.1 Refer to lines 1–3 (‘I made this … in my cabinet’).
- What compelled Dr Jekyll to create Edward Hyde? State TWO points. (2)
- Discuss what is ironic about the choices Dr Jekyll makes concerning Edward Hyde. (2)
2.2.2 Refer to lines 3–4 (‘For two months … to my determination’).
- Explain what Dr Jekyll means when he says, ‘I was true to my determination for two months’. (1)
- Explain why the following statement is FALSE.
Dr Jekyll decides to stay true to his determination for two months because he is done being Edward Hyde. (1)
2.2.3 Refer to lines 18–19 (‘My devil had … came out roaring’).
- Identify the figure of speech in these lines. (1)
- Explain how this figure of speech is relevant in this extract. (2)
2.2.4 With reference to the novel, compare the characters of Dr Jekyll and Edward Hyde. (2)
2.2.5 One of the themes evident in this novel is the importance of reputation. Discuss how this theme is revealed through Dr Jekyll. (3)
2.2.6 Dr Jekyll shows signs of a drug addict from the beginning of the novel. Discuss your view. (3)
[35]
TOTAL SECTION A: 35
SECTION B: DRAMA
In this section, there are questions set on the following dramas:
- MACBETH by William Shakespeare
- MY CHILDREN! MY AFRICA! by Athol Fugard
Answer the questions on the drama that you have studied.
QUESTION 3: MACBETH
Read the extracts from the play below and answer the questions set on each. The number of marks allocated to each question serves as a guide to the expected length of your answer.
NOTE: Answer the questions set on BOTH extracts, i.e. QUESTION 3.1. AND QUESTION 3.2.
3.1 EXTRACT E
[Macbeth (as King) has a banquet with the Lords.]
ROSS: His absence, sir [Act 3, Scene 4] |
3.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (3.1.1(a)–3.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
|
(4 x 1) (4)
3.1.2 Refer to lines 1–2 (‘His absence, sir … upon his promise’).
- Explain what Ross means in these lines. (2)
- What is the real reason why Banquo is not at the banquet as he promised? (1)
3.1.3 Refer to line 11 (‘Thou canst not say I did it’).
- What does ‘it’ refer to in this line? (1)
- Explain why Macbeth decides to kill Banquo.
State TWO points. (2)
3.1.4 Choose the correct answer to complete the following sentence.
Write only the letter (A–D) next to the question number (3.1.4) in the ANSWER BOOK.
Refer to line 12 (‘Thy gory locks at me’).
‘Gory locks’ are/is …
- dreadlocks.
- bloodstained hair.
- curly hair.
- attractive long hair. (1)
3.1.5 Refer to line 19 (‘Are you a man?’).
- What tone would Lady Macbeth use in this line? (1)
- Why would Lady Macbeth use this tone in this line? (1)
3.1.6 Refer to line 31 (‘Prithee, see there! ... How you say?’).
If you were the director of this play, what would you tell Macbeth to do when saying this line?
State TWO points. (2)
3.1.7 Macbeth is not an evil person; circumstances force him to kill. Discuss your view. (3)
AND
3.2 EXTRACT F
[Enter Ross, bringing the news from Scotland.]
MACBETH: Where are they? Gone? Let this pernicious hour Stand aye accursed in the calendar! [Act 4, Scene 1] |
3.2.1 Refer to lines 1–2 (‘Where are they? … in the calendar’).
- Identify the figure of speech in these lines. (1)
- Explain why this figure of speech is appropriate in these lines. (2)
3.2.2 Refer to line 11. (‘And damned all those that trust them’). With reference to the play discuss the irony in this statement. (2)
3.2.3 Refer to lines 17–19 (‘Time, thou anticipatest … go with it’). What does Macbeth mean in these lines? (2)
3.2.4 Explain why Macduff fled to England. State TWO points. (2)
3.2.5 What does this extract reveal about the character of Macbeth? Substantiate your answer. (2)
3.2.6 One of the themes in the play is guilt and its consequences. Discuss how this theme is relevant to Macbeth and Lady Macbeth. (3)
3.2.7 Macbeth’s anger towards Macduff is justified. Discuss your view. (3)
[35]
QUESTION 4: MY CHILDREN! MY AFRICA!
Read the extracts from the play below and answer the set questions. The number of marks allocated to each question serves as a guide to the expected length of your answer.
NOTE: Answer the questions set on BOTH extracts, i.e. QUESTION 4.1 AND QUESTION 4.2.
4.1 EXTRACT G
[The great debate.]
MR M: Order please! [Act 1, Scene 1] |
4.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (4.1.1(a)–4.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
|
(4 x 1) (4)
4.1.2
- In your own words, state the topic debated by Thami and Isabel. (1)
- Explain how Isabel wins the debate. (2)
4.1.3 With reference to this extract, state TWO points for the use of the ellipses in lines 3–7. (2)
4.1.4 Choose the correct answer to complete the following sentence. Write only the letter (A–D) next to the question number (4.1.4) in the ANSWER BOOK.
‘Intuitive’ in line 7 means …
- instinctive.
- fearless.
- respected.
- intimidated. (1)
4.1.5 Refer to lines 20–22 (‘Enthusiasm for your … but no harness’).
- Identify the figure of speech in this line. (1)
- Explain why this figure of speech is appropriate in these lines. (2)
4.1.6 What does this extract reveal about the character of Mr M? Substantiate your answer. (2)
4.1.7 Dialogue and personal discipline are a better solution than violence. Discuss your view. (3)
AND
4.2 EXTRACT H
[Thami refuses to learn.]
THAMI: Do you understand now why it is not as easy as it used to be to sit behind that desk and learn only what Oom Dawie has decided I must know? My head is rebellious. It refuses now to remember when the Dutch landed, and the Huguenots landed, [Act 1, Scene 6] |
4.2.1 State TWO points why Mr Grobbelaar insists that the learners call him ‘Oom Dawie’. (2)
4.2.2 Explain the difference in learning about the ‘arrival of the Huguenots’ and learning about ‘Kliptown in 1955’ according to Thami. (2)
4.2.3 Refer to line 11 (‘We don’t need Zolile classrooms anymore’).
- What tone would Thami use in this line? (1)
- Why would Thami use this tone in this line? (1)
4.2.4 Explain why the following statement is FALSE. Mr Grobbelaar is the principal at Zolile High School. (1)
4.2.5 With reference to the drama, what is ironic about Thami finding another school where people whisper? (2)
4.2.6 Refer to lines 13–14 (‘No, good people. We have woken up at last’).
If you were the director of this play, what would you tell Thami to do while saying these lines? State TWO points. (2)
4.2.7 One of the themes in the play is the meaning of a useful life.
Discuss how this theme is relevant to Mr M and Isabel. (3)
4.2.8 Thami declares Bantu Education as completely useless. Discuss your view. (3)
[35]
TOTAL SECTION B: 35
SECTION C: SHORT STORIES
In this section questions are set on the following short stories:
- A CHIP OF GLASS RUBY by Nadine Gordimer
- VILLAGE PEOPLE by Bessie Head
QUESTION 5
Read the extracts from the TWO short stories and answer the questions set on each. The number of marks allocated serves as a guide to the expected length of your answer.
NOTE: Answer the questions set on BOTH extracts, i.e. QUESTION 5.1 AND QUESTION 5.2.
5.1 ‘A CHIP OF GLASS RUBY’
EXTRACT I
[Mrs Bamjee gets arrested.]
| The man held it away from her.The man held it away from her.‘What does it matter, Ma?’It was true that no one in the house had ever read it; but she said, ‘It’s for my children.’-‘Ma leave it.’ Jimmy who was squat and plump, looked like a merchant advising a client against a roll of silk she had set her heart on.She went into the bedroom and got dressed. When she came out in her old yellow sari with a brown coat over it, the faces of the children were behind her like faces on the platform at a railway station. They kissed her good-bye. The policemen did not hurry her, but she seemed to be in a hurry just the same.‘What am I going to do?’ Bamjee accused them all.The police looked away patiently.‘It’ll be all right. Girlie will help. The big children can manage. And Yusuf’ – The children crowded in around her; two of the younger ones had awakened and appeared, asking shrill questions. ‘Come on,’ said the policemen.‘I want to speak to my husband.’ She broke away and came back to him, and the movement of her sari hid them from the rest of the room for a moment. |
5.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (5.1.1(a)–5.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
|
(4 x 1) (4)
5.1.2 Refer to line 1 (‘The man held it away from her’).
- Choose the correct answer to complete the following sentence. Write only the letter (A–D) next to the question number (5.1.2) in the ANSWER BOOK.
‘it’ in this sentence refers to a …- duplicating machine.
- pamphlet.
- ruby.
- tome. (1)
- Explain why this ‘it’ is important to Mrs Bamjee. Mention TWO points. (2)
5.1.3 With reference to the story, why is it ironic that the coloured policemen come to arrest Mrs Bamjee? (2)
5.1.4 Explain why the following statement is FALSE. Mrs Bamjee was arrested because she joined a hunger strike in prison. (1)
5.1.5 Refer to line 10 (‘What am I going to do?’).
- What tone would Mr Bamjee use in this line? (1)
- Why would Mr Bamjee use this tone in this line? (1)
- What does this line tell us about the character of Mr Bamjee? Substantiate your answer. (2)
5.1.6 Mrs Bamjee is involved in the political affairs of black people that have nothing to do with her. Discuss your view. (3)
5.2 ‘VILLAGE PEOPLE’
EXTRACT J
[The narrator assists an old lady.]
| Yet she seemed so strong that it was a shock when she suddenly bent double, retched and coughed emptily, and crumbled to the ground like a quiet sigh. ‘What is it, Mmm? ‘What is the matter?’ I asked. ‘Water, water,’ she said faintly. ‘Wait a minute. I shall ask at this hut here if there is any water.’ ‘What is the matter?’ they asked. ‘The old lady is ill,’ I said. ‘No,’ she said curtly. ‘I am not ill. I am hungry.’ The crowd laughed in embarrassment that she should display her need so nakedly. They turned away; but old ladies have no more shame left. They are like children. They give way to weakness and cry openly when they are hungry. ‘Never mind, ‘I said. ‘Hunger is a terrible thing. My hut is not far away. This small child will take you. Wait till I come back, then I shall prepare food for you.’ Then it was late afternoon. The old lady had long passed from my mind when a strange young woman, unknown to me, walked into the yard with a pail of water on her head. She set it down outside the door and squatted low. ‘Good-day. How are you?’ I said. She returned the greeting, keeping her face empty and carefully averted. It is impossible to say: what do you want? |
5.2.1 Refer to lines 2–3 (‘retched and coughed … a quiet sigh’).
- Identify the figure of speech in this line. (1)
- Explain why this figure of speech is appropriate in this line. (2)
5.2.2 Explain the crowd’s reaction to the old lady. (2)
5.2.3 How does the narrator help the old lady? State TWO points. (2)
5.2.4 In your own words explain the meaning of ‘display her need so nakedly’. (1)
5.2.5 Why does the old lady’s family repay the narrator with a bucket of water? (2)
5.2.6 With reference to the story, compare the character of the old woman and the young woman (who brings her water). (2)
5.2.7 One of the themes in the short story is kindness and compassion. Discuss how this theme is relevant to the village people in this story. (3)
5.2.8 In this story, the narrator states that poverty-stricken people ‘are not outgoing’ and open to change. Discuss your view. (3)
[35]
TOTAL SECTION C: 35
SECTION D: POETRY
In this section, questions are set on the following poems:
- ‘Captive’ by Francis C. Slater
- ‘Mid-term break’ by Seamus Heaney
NOTE: Answer the questions set on BOTH poems, i.e. QUESTION 6.1 AND QUESTION 6.2.
QUESTION 6
6.1 Read the poem carefully and then answer the questions which follow. The number of marks allocated to each question serves as a guide to the expected length of your answer.
Captive – Francis C. Slater Lament of a sick Xhosa mine-labourer in a compound hospital
|
6.1.1 Choose a description from COLUMN B that matches a name in COLUMN A. Write only the letter (A–E) next to the question numbers (6.1.1(a)–6.1.1(d)) in the ANSWER BOOK.
| COLUMN A | COLUMN B |
|
(c) C a creature’s home or hiding place (d) D a prisoner or someone in jail E a trap for animals |
(4 x 1) (4)
6.1.2 Refer to lines 1–2 (‘As a wild … dun-coloured cow’).
- Identify the figure of speech in line 1. (1)
- Explain why this figure of speech is relevant in the poem. (2)
6.1.3 Refer to line 5 (‘And burn and shiver while I listen to the buzzing’).
Choose the correct answer to complete the following sentence.
Write only the letter (A–D) next to the question number (6.1.3) in the ANSWER BOOK.
‘Buzzing’ in this line is an example of a(n) …
- antithesis.
- apostrophe.
- onomatopoeia.
- oxymoron. (1)
6.1.4 Explain what is meant by, ‘And chew the juicy cud of gathered day’ in line 26. (2)
6.1.5 Give TWO examples which show that the poet has some knowledge of rural life. (2)
6.1.6 One of the themes evident in this poem is the evils of migrant labour. Discuss the theme in the context of the poem. (3)
6.1.7 The condition of the compound hospital in which the speaker finds himself justifies his longing for home. Discuss your view. (3)
AND
6.2 Read the poem carefully and then answer the questions which follow. The number of marks allocated to each question serves as a guide to the expected length of your answer.
Mid-term break – Seamus Heany
|
6.2.1 Refer to line 2 (‘counting bells knelling classes to a close’). Why is the bell described as ‘knelling’? (1)
6.2.2 Refer to line 5 (‘He had always taken funerals in his stride’). Explain what is meant by ‘taken funerals in his stride’. Use your OWN words. (2)
6.2.3 In line 7 the ‘baby cooed and laughed’. With reference to the poem, what does this suggest about the baby? (2)
6.2.4 Refer to line 10 (‘And tell me they were sorry for my trouble’).
- Identify the tone in this line. (1)
- Why would the old men use this tone in this line? (1)
6.2.5 Explain why the following statement is FALSE. The speaker sees his brother for the first time in six weeks because the brother has been in hospital. (1)
6.2.6 Refer to stanza 5 (‘In hers and … by the nurses’). Quote ONE word to show that the nurses had tried to stop the boy’s bleeding before he died. (1)
6.2.7 Refer to line 20 (‘He lay in the four foot box as in his cot’).
- Identify the figure of speech in this line. (1)
- Explain why this figure of speech is relevant in this poem. (2)
6.2.8 Explain the irony in the title of the poem. (2)
6.2.9 Tragic death is more painful to adults than it is to children. Discuss your view. (3)
[35]
TOTAL SECTION D: 35
GRAND TOTAL: 70
English First Additional Language Paper 1 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
INSTRUCTIONS AND INFORMATION
- This question paper consists of THREE sections:
SECTION A: Comprehension (30)
SECTION B: Summary (10)
SECTION C: Language (40) - Answer ALL the questions.
- Read ALL the instructions carefully.
- Start EACH section on a NEW page.
- Leave a line after each answer.
- Number the answers correctly according to the numbering system used in this question paper.
- For multiple-choice questions, write only the letter (A–D) next to the question number in the ANSWER BOOK
- Pay special attention to spelling and sentence construction.
- Use the following time frame as a guideline:
SECTION A: 50 minutes
SECTION B: 20 minutes
SECTION C: 50 minutes - Write neatly and legibly.
QUESTIONS
SECTION A: COMPREHENSION QUESTION 1
Read BOTH TEXT A and TEXT B and answer the set questions.
TEXT A
HIGH SCHOOL LEARNERS SHOULD PLAY SPORT
- It was mostly athletics that played a major role in high school sport for decades. Today, the field of sport has grown, with a great variety of competitive options for male and female learners alike. Many learners get involved in high school athletics for the love of it, but there are important benefits from extramural activities that learners and parents may not even realise.
- According to recent studies, learners who participate in high school sports learn the benefit of representing their community on the field or court. These athletes learn the fun of competition and enjoy the praise of a job well done for their school. This feeling of community and the honour of representing the home team may lead to great success as professional clubs are always looking for new players.
- The fitness level of athletes in high school sports programmes cannot be underestimated. According to a 2006 study it was found that when female learners participate in athletics in high school, their weight and body mass improve. A 2001 survey found that learners agreed they would not spend as much time sitting, watching television and playing video games if they played sport.
- Studies also suggest that learners who play sport are less likely to participate in unhealthy or risky behaviour. A 2002 study found that learners who did not
take part in extramural activities in high school were 49 percent more likely to use drugs and 37 percent more likely to become teen parents. The survey also showed that learners who do not play sport stayed absent from school more. - Another study published in the journal, Medicine & Science in Sports and Exercise in August 2007, found that learners who were active in sports like
soccer, football and even skateboarding performed ten percent better in core subjects like Mathematics, Science and Languages. - Athletes learn lessons that go beyond the classroom like determination, patience and practice. Team members learn that practice is required, even when they would prefer to be spending time with friends. They learn the harder they work, the better they perform. They also discover that by never giving up, they are more likely to achieve their goals. These life lessons benefit learners long after the high school years, helping them succeed after school.
- In team sport everyone is working towards a common goal, so learners learn first-hand how their performance affects the rest of the team.
- Sports teams have many positive mentors, from the coaches on the side lines to the leaders on the team. Learners learn to work with a wide range of expert figures, who teach them important lessons about hard work, respect and good sportsmanship. Early experiences with mentors like these help shape athletes in positive ways for the rest of their lives.
- Learners who participate in sports often form close friendships with others on the team. These relationships are essential for mental, emotional and physical health throughout the high school years. Learners bond together over a common passion, and the time they spend together at practice and games builds tight bonds that often last long after high school is over.
- As learners advance through the ranks of the high school team, they learn valuable leadership skills. Senior athletes are expected to encourage younger team members and hold them accountable. They set an example and often provide advice and guidance both on and off the field.
- A mindset for success is instilled through time management skills among other things. Learners who play sport must find creative ways to improve and strengthen their internal skills for handling pressure. They must learn when to take risks and take responsibility for individual performance. To improve on the sports field, they need to develop their focus and concentration.
- Practice and games take up plenty of a learner’s time, leaving much less for schoolwork and other activities. Athletes must learn time management skills if
they are to get everything finished. One learner said, “It definitely helps with time management. I know when I must do my schoolwork, and when I must practice.” - These skills go far beyond the sports field or even beyond high school. Learners who play sport reap the benefit of their training for the rest of their lives.
[Adapted from www.publicschoolreview.com, 2019]
1.1 Refer to paragraph 1.
1.1.1 State ONE reason why it is important to play sport in high school. (1)
1.1.2 Explain the difference between high school sport in the past and high school sport today. (2)
1.2 Refer to paragraph 3.
What would learners do if they did not play sport? Mention ONE fact. (1)
1.3 Refer to paragraph 4.
1.3.1 Explain why the writer mentions 37 percent and 49 percent. (2)
1.3.2 Why do you think that ‘learners who do not play sport stay absent from school more’? (2)
1.4 Refer to paragraph 5.
1.4.1 Why are the words ‘Medicine & Science in Sports and Exercise’ written in italics? (1)
1.4.2 Quote FOUR consecutive words which show that playing sport improves learners’ marks. (1)
1.5 Refer to paragraph 6.
1.5.1 What does the writer mean by, ‘lessons that go beyond the classroom’? (1)
1.5.2 How can learners use ‘determination, patience and practice’ to perform better at school? (2)
1.6 Choose the correct answer to complete the following sentence.
Mentors (paragraph 8) are people who … others.
- follow
- guide
- mislead
- abuse (1)
1.7 Refer to paragraph 9.
Why is the following statement FALSE?
Learners who play for the same team are competitive and never become friends. (1)
1.8 Refer to paragraph 10.
Explain how learners can use their leadership skills on the sports field. (2)
1.9 Using your OWN words, explain what is meant by ‘time management skills’. (1)
1.10 Why does the writer refer to studies that have been conducted? (2)
1.11 Do you agree that taking part in sport will advantage you for life? (2)
1.12 Discuss the suitability of the title, ‘HIGH SCHOOL LEARNERS PLAY SPORT’. SHOULD (2)
TEXT B
SHINE LITERACY PROGRAMME Currently 1124 volunteers deliver Shine Literacy Programmes to 7 413 children at [Adapted from www.shineliteracy.org.za] |
1.13 Which word tells us that people do not get paid to work for the Shine Literacy Organisation? (1)
1.14 How many more provinces can be reached through this programme? (1)
1.15 ‘Words can change worlds’. Do you think this can be said about the boy in the picture? Give reasons for your answer. (2)
1.16 Discuss whether the Shine Literacy Programme is an important one for South African children. (2)
TOTAL SECTION A: 30
SECTION B: SUMMARY
QUESTION 2
Cell phone manners is important but ignored by many.
Read TEXT C below and list SEVEN tips on cell phone manners.
INSTRUCTIONS
- Your summary must be written in point form.
- List your SEVEN points in full sentences, using no more than 70 words.
- Number your sentences from 1 to 7.
- Write only ONE point per sentence.
- Use your OWN words as far as possible.
- Indicate the number of words you have used in brackets at the end of your summary.
TEXT C
CELL PHONE MANNERS Do not be caught committing cell phone sins. Follow these rules to avoid being rude and annoying. [Adapted from www.rd.com] |
TOTAL SECTION B: 10
SECTION C: LANGUAGE
QUESTION 3: ANALYSING AN ADVERTISEMENT
Study the advertisement (TEXT D) below and answer the set questions.
TEXT D
[Adapted from www.arrivealive.co.za]
3.1 At which target audience is the above message aimed? (1)
3.2 What do the words ‘Fatal Accidents’ show? (2)
3.3 Choose the correct answer:
Which government department is responsible for spreading this message?
- The Department of Education
- The Department of Agriculture
- The Department of Transport
- The Department of Labour (1)
3.4 Give TWO reasons why it is not a good idea to drink and drive. (2)
3.5 Why do you think the message above is an important one for our country? (2)
3.6 Do you think the advertisement is successful in conveying its message? (2) [10]
QUESTION 4: ANALYSING A CARTOON
Read the cartoon (TEXT E) below and answer the set questions.
TEXT E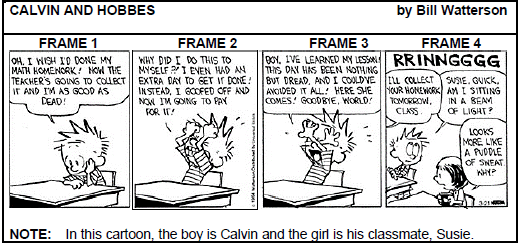
4.1 Write the word ‘Math’ in FRAME 1 in full. (1)
4.2 Describe Calvin’s body language in FRAME 2. (2)
4.3 How do you think Calvin is ‘going to pay’ for not doing his homework? Mention TWO facts. (2)
4.4 Suggest TWO reasons why Calvin is no longer worried about not doing his homework in FRAME 4. (2)
4.5 Which word does Susie use to indicate that Calvin panics? (1)
4.6 Do you find this cartoon humorous? Substantiate your answer. (2) [10]
QUESTION 5: LANGUAGE AND EDITING SKILLS
5.1 Read the passage (TEXT F) below, which has some deliberate errors, and answer the set questions.
TEXT F
PUT FOOT FOUNDATION
[Adapted from www.putfootfoundation.org.za] |
5.1.1 Correct the SINGLE error in EACH of the following sentences. Write down ONLY the question numbers and the words you have corrected.
- We belief that there is no greater experience than donating shoes.(1)
- That is why we invite and strong encourage our donors to take part in this life-changing experience.(1)
- By doing this, we restore hope, proud and dignity. (1)
- We want to reach each of our countries neediest learners. (1)
5.1.2 Give the correct form of the underlined word. The children are provided with comfort shoes. (1)
5.1.3 Complete the following tag question. Write down ONLY the missing words. Donors physically place shoes onto the feet of every child, … …? (1)
5.1.4 Combine the following sentences into a single sentence by using neither … nor.
Donors drop the shoes.
Donors leave immediately. (1)
5.1.5 Provide a homophone for the underlined word: Africa’s rough and unforgiving terrain is tough on children’s soles. (1)
5.1.6 Rewrite the following sentence in the future continuous tense: The children’s eyes light up when they receive their shoes. (1)
5.1.7 Rewrite the following sentence in the negative form: A donor ensures that every child’s shoe fits. (1)
5.1.8 Rewrite the following sentence in reported speech: The organisers said, ‘Shoes are vital to these children and it makes them happy.’ (4)
5.1.9 Rewrite the underlined words as a contraction. They have enriched many lives through their donation of shoes. (1)
5.2 Study the text (TEXT G) below and answer the set questions.
TEXT G
[Source: www.capestylemag.com]
5.2.1 Rewrite the following sentence in the passive voice: We should help those in need. (1)
5.2.2 Study the following sentence: If you cannot give money or goods, give of your time. State the part of speech of the underlined words. (2)
5.2.3 Rewrite the following sentence in the singular form. Giving unwanted items to the needy should be a priority. (1)
5.2.4 Give the correct degree of comparison in the following sentence: Giving to others is the (satisfying) feeling. (1)
[20]
TOTAL SECTION C: 40
GRAND TOTAL: 80
Mathematical Literacy Paper 2 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
Symbol | Explanation |
M | Method |
M/A | Method with accuracy |
MCA | Method with consistent accuracy |
CA | Consistent accuracy |
A | Accuracy |
C | Conversion |
S | Simplification |
RT/RG/RM | Reading from a table OR Reading from a graph OR Read from map |
F | Choosing the correct formula |
SF | Substitution in a formula |
J | Justification |
P | Penalty, e.g. for no units, incorrect rounding off etc. |
R | Rounding off OR Reason |
AO | Answer only |
NPR | No penalty for rounding |
MARKING SCHEME
QUESTION 1 [37] | |||
Ques. | Solution | Explanation | Level |
1.1.1 | Amount used for 3 batches = 3 × 125 MA |
| M L2 |
1.1.2 | Price of 3 eggs = 14,99 M |
| F L3 |
1.1.3 | °Fahrenheit = 1,8 × °Celsius + 32° |
| M L2 |
1.1.4 | Time taken for 9 batches = 25 min + 45 min |
| M L4 |
1.1.5 | Cake flour = 3 × 250 = 150 grams Invalid O OR Cake flour = 3 × 250 = 150 grams Invalid O |
| F&M L4 |
1.2.1 | 7 feet 8 inches = (7 × 0,3048) + (8 × 0,0254) C 6 feet 6 inches = (6 × 0,3048) + (6 × 0,0254) Length = 2,3368 m + 1,9812 m OR Total feet in metres = 7 feet + 6 feet M |
| M L3 | |
1.2.2 | Top view A |
| M& P | |
1.2.3 |
|
| M& P L4 | |
QUESTION 2 [39] | |||
Ques. | Solution | Explanation | Level |
2.1.1 | Simple Interest |
| F L2 |
2.1.2 | Compound Interest OR Amount after 35 months She will pay less interest on Option 2 (compound interest) |
| F L4(1) |
2.2.1 | Factory 1 M Factory 2 |
| D L3(5) L4(3) |
2.2.2 | No, factory 2 is still new A |
| D L4 |
2.3.1 | Percentage = 94 MA × 100% |
| M L2 |
2.3.2 | Over the length = 310 ?? |
| M L4 | |
2.4.1 | Northeast |
| M&P L2 | |
2.4.2 | Probability = 1A |
| P L2 | |
2.4.3 |
|
| M&P L4 | |
QUESTION 3 [36] | |||
Ques. | Solution A | Explanation | Level |
3.1.1 | Initial tax = 0,18 × 1958A50 |
| F L2 |
3.1.2 | Pension = 7,5 × 37 537,75 MA |
| F L3 |
3.1.3 | Taxable income = (37 537,75 × 12) – R40 683,98 |
| F L2 |
3.1.4 | Tax payable = 63 853 + 31% of taxable income above 305 850 |
| F L3&4 |
3.1.5 | They receive 3 rebates |
| F L4 |
3.1.6 | Gross monthly salary in 2018/2019 = 37 537,75 A |
| F V2 |
3.2.1 | Value of A = 90 – (8 + 13 + 30 + 15 + 10) |
| D L2 |
3.2.2 | 16 years A |
| D L2 |
3.2.3 | Average age MCA |
| D L3 |
3.2.4 | Number of boys = 13 + 30 + 15 + 7 |
| P L2 |
3.2.5 | The weight of the boys should also be taken into account.A |
| D L2 |
QUESTION 4 [38] | |||
Ques. | Solution | Explanation | Level |
4.1.1 | 14,202957 A R The lower the value, the stronger the Rand |
| D L2&4 |
4.1.2 | Amount after exchange fee = 40 830 – (40 830 × 0,045) At 14,983385 = 38 992,65 MCA OR At 14,983385 = 40 830 MA |
| F L3&4 |
4.1.3 |
Accept any other relevant responses |
| D L4 |
4.2.1 | Enlargement: Map: |
| L4 | ||
4.2.2 | Traveller 1 and 2 = $670,36 × 2 |
| F | ||
4.3.1 | Range = Highest value – Lowest value |
| D L2 | ||
4.3.2 | Probability = 5 A |
| D L2 | ||
4.3.3 | °Celsius = (°F – 32) ÷ 1,8 |
| M L2&4 | ||
| 4.3.4 |  |
| D L2 | ||
| TOTAL: | 150 | ||||
Final Examination Revision Papers - Physical Sciences Grade 10 Study Guide
Physics Examination (Paper 1)
Data for Physical Sciences Grade 10 Physics (Paper 1)
Physical constants
Name | Symbol | Value |
Acceleration due to gravity | g | 9,8 m.s–2 |
Speed of light in a vacuum | c | 3,0 × 108 m.s–1 |
Charge on electron | e– | –1,6 × 10–19 C |
Planck’s constant | h | 6,63 × 10–34 J.s |
Formulae
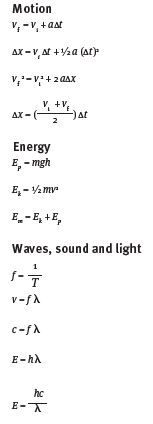

SECTION A
QUESTION 1: ONE-WORD ANSWERS
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
1.1 The rate of change of position.
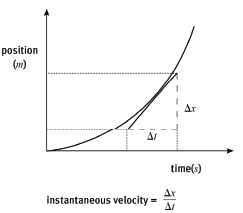
1.2 The quantity represented by the area under a velocity vs. time graph.
1.3 The type of wave in which the vibrations of the particles is at right angles to the direction in which the wave is travelling.
1.4 The way in which a voltmeter is connected in a circuit.
1.5 A quantum of visible light. [5]
QUESTION 2: MATCHING PAIRS
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 the principle of adding together the amplitudes of waves that meet, so as to find the resultant amplitude 2.2 the highest frequency of electromagnetic radiation 2.3 magnetic field lines 2.4 gradient of a velocity vs. time graph 2.5 kinetic energy of an object falling in the absence of air friction |
|
[5]
QUESTION 3: TRUE/FALSE
3.1 The electrical resistance of a conductor depends only on the length and the thickness of the conductor.
3.2 For a car travelling from rest and at constant acceleration, its displacement is directly proportional to its time of travel.
3.3 When resistors are connected in series they act as dividers of potential difference.
3.4 The area under a velocity vs. time graph represents the acceleration of the object.
3.5 When an object falls vertically in the absence of air friction, its mechanical energy remains constant. 5 × 2 = [10]
QUESTION 4: MULTIPLE CHOICE
4.1 In which of the following situations will the distance covered and the magnitude of the displacement of a car be the same?
- A girl runs in a straight line across a level field.
- A car travels half the circumference of a circular race track.
- A car travels from Cape Town to Johannesburg.
- A car travels the full circumference of a circular race track. (3)
4.2 Consider the accompanying velocity–time graph of a child riding her bicycle.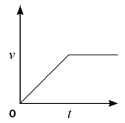
The corresponding graph, representing the acceleration of the bicycle vs. the time is: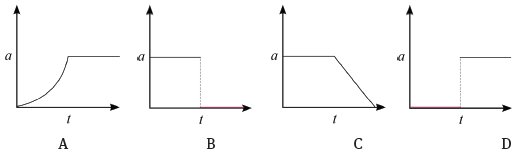 (3)
(3)
4.3 A bungi jumper of mass m falls freely from a bridge of height h. He reaches a velocity v after falling a distance x. If air resistance is negligible, his total mechanical energy at this point is:
- mg(h–x).
- ½ mv2.
- mgh + ½ mv2.
- mgh. (3)
4.4 When resistor R1 and R2 are connected in parallel, the total resistance of the combination is always:
- very much larger than either R1 or R2.
- equal to R1 + R2.
- equal to R1 × R2.
- smaller than the smaller of R1 and R2. (3)
4.5 A source of frequency 500 Hz emits waves of wavelength 0,2 m. How long does it take the waves to travel 300 m?
- 3 s
- 12 s
- 60 s
- 100 s (3) [15]
SECTION B
QUESTION 1
1.1 Distinguish between a vector quantity and a scalar quantity. (2)
1.2 What is meant by the ‘resultant’ of two forces? (2)
1.3 A box, on the floor, is being pulled by two boys by means of ropes tied to the box. The forces that they apply are 70 N and 50 N. What is the minimum resultant force that the ropes can exert on the box, and what must be the angle between the ropes in order for them to exert this minimum force? (2) [6]
QUESTION 2
The two boys apply the same forces as in question 1 on the box by means of ropes. The 70 N force is exerted in a direction 90°, while the 50 N force is exerted in a direction 180°.
2.1 By means of an accurate scale drawing, using the tail-to-tail method, determine the magnitude and direction of the resultant force being exerted on the box. (Use a scale of 10 mm = 10 N.) (7)
2.2 Verify your answer by using mathematical and trigonometrical calculations to determine the resultant force. (6) [13]
QUESTION 3
The brakes of a car are being tested on a straight, level tarred road. At the far end of the road is a stretch of soft sand to stop the car, should it not stop in time. The length of the road up to the sand is 192 m. The car accelerates uniformly at 9,8 m.s–2 from rest at the start of the road. After travelling 122,5 m, the brakes are applied and the car slows down uniformly, coming to rest 10 m from the sand.
3.1 Define acceleration. (2)
3.2 Calculate the time taken for the car to travel the first 122,5 m. (4)
3.3 What is the velocity of the car at the 122,5 mark? (4)
3.4 Calculate the acceleration of the car during the time that the brakes are applied. (5)
3.5 The speedometer of a car registers ‘instantaneous speed’. How is instantaneous speed measured? (3) [18]
QUESTION 4
The following velocity–time graph represents the motion of a trolley on a track. In carrying out the re- quired calculations do not use equations of motion.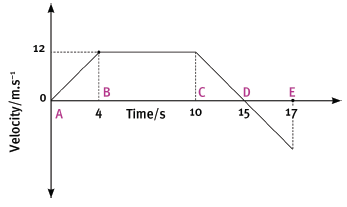
4.1 Calculate from the graph the acceleration of the trolley for the time interval CD. (4)
4.2 Describe (in words) the motion of the trolley for the interval ABC. (3)
4.3 What happened to the trolley during time interval DE? (3)
4.4 Calculate the displacement of the trolley during the interval ABC. (5)
4.5 Draw a sketch of the corresponding acceleration–time graph. Show the time values (0 s to 17 s) on the time axis, but do not show any acceleration values on the Y-axis. (6) [21]
QUESTION 5
Moneeb and his little sister Moneeba are playing with a toy car of mass 3 kg on the slide in the school playground. The toy car is moving at a speed of 2,63 m.s–1 when it passes point P. Point P is 0,75 m above point R, the lowest position of the slide. Ignore all types of friction. Express answers correct to two decimal places.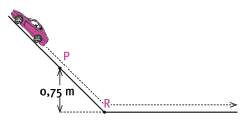
5.1 State what is meant by the ‘mechanical energy’ of an object. (2)
5.2 Under what condition is the mechanical energy of a falling object conserved? (1)
5.3 Calculate the total mechanical energy of the car at point P. (6)
5.4 What is the kinetic energy of the car at point R? (2)
5.5 Calculate the velocity of the car at point R. (4) [15]
QUESTION 6
Two pulses, P and Q in a string, approach each other at the same speed. Pulse P has an amplitude of +4,0 cm when it is at position X. Pulse Q has an amplitude of –6,0 cm when it is at position Z. Points X and Z are the same distance from point Y. The pulses both have a length of 8,0 cm. Pulses P and Q meet at position Y. Assume that no energy is lost.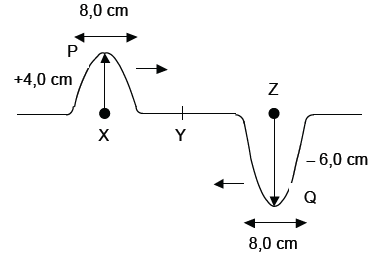
6.1 Write down the definition of a pulse. (2)
6.2 Make a labelled sketch to show what happens when the pulses P and Q meet at position Y. Also indicate the pulse length. (4)
6.3 A transverse wave is formed by a succession of pulses each with amplitude 4 cm and pulse length 8 cm (similar to P above). If the period of this transverse wave is 0,4 s, calculate the
velocity of the wave in m.s–1. (6) [12]
QUESTION 7
Water waves are travelling towards the concrete wall of a harbour, as shown in the sketch below. Six wavelengths strike the wall in 24 seconds. The distance between successive crests is 10 m. The height of the waves from trough to crest is 2,5 m.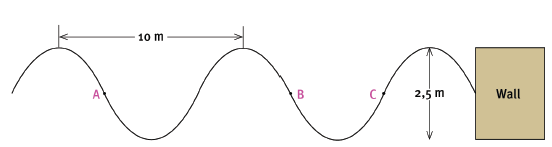
7.1 Are points A and C ‘in phase’? Explain your answer. (3)
7.2 The waves are moving towards the wall, from left to right. In what direction is point B on the wave moving? (2)
7.3 What is the amplitude of the wave? (2)
7.4 Calculate the period of the wave. (3)
7.5 Calculate the frequency of the wave. (3) [13]
QUESTION 8
Pianos, guitars and other stringed musical instruments have to be ‘tuned’. This means that the tension in the strings must be adjusted so that they produce the correct note when struck or plucked. This is done by matching the sound produced by the instrument to that produced by a ‘tuning fork’. A particular tuning fork is marked 440 Hz.
8.1 What is meant by the marking 440 Hz? (2)
8.2 What type of wave is a sound wave? (2)
8.3 The speed of sound at sunrise at a particular place is 330 m.s–1. Would you expect the speed to be faster or slower at midday when it is much hotter? (2)
8.4 When sound travels from air into water, what happens to the speed of the sound? Give a reason for you answer. (4) [10]
QUESTION 9
When we see a rainbow, we are seeing a small part of a very wide range of wavelengths called an ‘electromagnetic spectrum’.
9.1 What are the names of the portions of the electromagnetic spectrum on either side of the visible spectrum? (2)
9.2 Explain what contribution Max Planck made to our understanding of electromagnetic waves? (4)
9.3 Explain why visible light is considered to have a ‘dual nature’. (6)
9.4 The energy of a quantum of electromagnetic radiation is 3,55 × 10–19 J. Calculate the wavelength of the wave. (5)
9.5 Express the answer to 9.4 in nanometres. (2) [19]
QUESTION 10
A plastic ruler is rubbed vigorously with a cloth. As a result, the ruler has become negatively charged. The ruler is then held close to a small bead covered with thin metal foil, as shown in the sketch.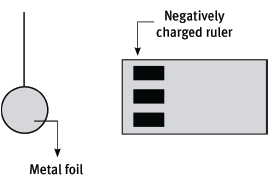
10.1 Why has the ruler become negatively charged? (2)
10.2 The charge on the ruler is –1,36 × 10–12 C. Calculate the number of excess electrons on the ruler. (4)
10.3 The bead is attracted by the ruler. Explain why it is attracted. (4)
10.4 What would you expect to see after the bead has touched the ruler? (2)
10.5 Explain why this should occur. (3) [15]
QUESTION 11
Consider the circuit diagram given below. You are given that V1 reads 12 V, A1 reads 3 A and V2 reads 9 V. Resistors R1 and R2 are identical.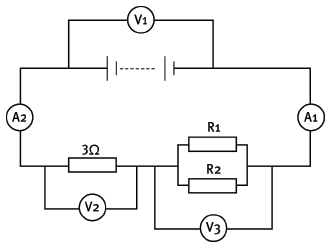
11.1 Define potential difference. (2)
11.2 What is the reading on V2? (2)
11.3 What is the reading on A2? (2)
11.4 You are given that the single-series resistor has a resistance of 3 Ω. Explain why the effective resistance of the parallel pair of resistors is 1 Ω. (4)
11.5 What is the resistance of R1? (2)
11.6 Calculate the charge that passes through the 3 Ω resistor in 2 minutes. (4) [16]
QUESTION 12
You are given this labelled circuit diagram.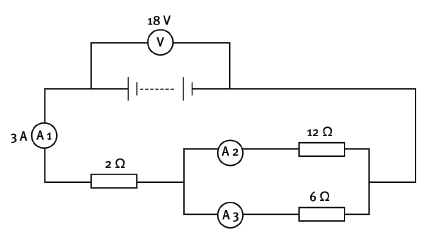
12.1 Calculate the equivalent resistance of the whole circuit. (5)
12.2 If the reading on A3 is 2 A, what is the reading on A2? (2) [7]
Answers
SECTION A
QUESTION 1: ONE-WORD ANSWERS
1.1 velocity
1.2 displacement
1.3 transverse
1.4 series
1.5 photon
QUESTION 2: MATCHING PAIRS
2.1 B
2.2 D
2.3 C
2.4 G
2.5 E
QUESTION 3: TRUE/FALSE
3.1 False. Electrical resistance depends on the type of material, length, thickness and temperature.
3.2 False. For a car travelling from rest and at constant acceleration, its displacement is directly proportional to the square of its time of travel.
3.3 True.
3.4 False.The area under a velocity vs. time graph represents the displacement of the object.
3.5 True.
QUESTION 4: MULTIPLE CHOICE
4.1 A
4.2 B
4.3 D
4.4 D
4.5 A
SECTION B
QUESTION 1
1.1 A vector quantity has both magnitude and direction, while a scalar quantity has magnitude only.
1.2 The resultant of a number of vectors is the single vector that has the same effect as all the vectors acting together.
1.3 20 N 180°
QUESTION 2
2.1 Scale: 10 mm = 10 N
(Correct length of 70 N)
(Correct length of 50 N)
Resultant force = 86 N (+ –2 N) 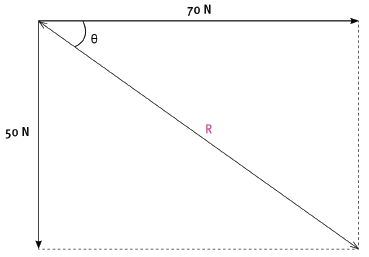
θ = 36° (+ –2°)
2.2 Using Pythagoras:
- R2 = 802 + 602
R = 86,0 N
Sin θ = opp = 50
hyp 86
θ = 35,6°
Direction = 125,6°
QUESTION 3
3.1 The rate of change of velocity.
3.2
- Δx = viΔt + ½ a(Δt)2
122,5 = 0 + ½ (9,8) (Δt)2
(Δt)2 = 122,5 = 25
4,9
Δt = 5 s
3.3
- vf = vi + aΔt OR vf2 = vi2 + 2 aΔ x
= 0 + (9,8) (5) = 0 + 2(9,8) (122,5)
= 49 m.s–1 = 2401
vf = 49 m.s–1
3.4 Distance travelled while brakes are applied
- = 192 – 122,5 – 10 = 59,5 m
vf2 = vi2 + 2 aΔx
0 = 492 + 2(a)(59,5)
a = −(492)
119
a = –20,2 m.s–2
3.5 By measuring the distance travelled during a very short time interval and applying the formula v = D/Δt
QUESTION 4
4.1 Acceleration =
- gradient = ∆y = 12
∆x 5
= –2,4 m.s–2
4.2 Accelerated uniformly from rest for 4 seconds then continued at constant velocity of 12 m.s–1 for a further 6 s.
4.3 Trolley reversed from rest with a uniform acceleration for 2 seconds.
4.4 Displacement = area under graph
- = ½ bh + lb
= ½ (4) (12) + (6) (12)
= 96 m
4.5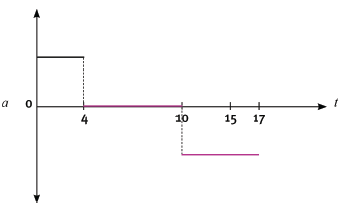
Graph 0 s – 4 s correct
Graph 4 s – 10 s correct
Graph 10 s – 17 s correct
QUESTION 5
5.1 The sum of the kinetic energy and potential energy.
5.2 zero friction
5.3 Mechanical energy
- = Ep + Ek
= mgh + ½ mv2
= 3 (9,8) (0,75) + ½ (3) (2,63)2
= 32,43 J
5.4 At R the Ep = 0. So Ek = EM = 32,43 J
5.5
- Ek = ½ mv2
v2 = 2 Ek = 2 (32,43) = 21,62
m 3
v = 4,65 m.s–1
QUESTION 6
6.1 A single disturbance in a medium
Pulse drawn correctly
Correct amplitude
Correct pulse length
6.3
- f = 1 = 1 = 2,5 Hz
T 0,4
Pulse length = 8 cm. Wavelength = 16 cm = 0,16 m
v = f λ = (2,5) (0,16)
= 0,4 m.s–1
QUESTION 7
7.1 No - They are not ‘in step’. When A is moving up, C is moving down.
7.2 Vertically upwards
7.3 1,25 m
7.4 If 6 wavelengths strike the wall in 24 s, one wavelength will strike every 4 s.
7.5
- f = 1 = 1
T 4
= 0,25 Hz
QUESTION 8
8.1 440 vibrations per second
8.2 longitudinal waves
8.3 faster
8.4 speed increases Water molecules are much closer together than air molecules, so the energy is transferred more quickly from molecule to molecule.
QUESTION 9
9.1
- infrared
ultraviolet
9.2
- Energy is not radiated continuously but in packages named ‘quanta’.
- The energy content of a quantum is directly proportional to the frequency of the wave.
9.2 A quantum of energy is similar to a particle, but within the quantum there is an electromagnetic wave. At the radio wave end of the spectrum, the wavelength is so long that the waves are almost continuous – the ‘wave nature’. At the gamma ray end the wavelength is so short that the wave nature is not noticeable – the quantum behaves as a particle. Visible light is in the middle of the whole spectrum, so both wave nature and particle nature are evident.
9.4
- E = hc λ = hc = (6,63 × 10–34) (3 × 108)
λ λ 3,55 × 10–19
= 5,6 × 10–7 m
9.5 560 nm
QUESTION 10
10.1 Electrons have been rubbed off the cloth, onto the ruler.
10.2
- Q = nqe n = Q = 1,36 × 10 – 12
qe 1,6 × 10 – 19
= 8,5 × 106 electrons
10.3 Loosely bound electrons in the metal foil are repelled to the far side of the bead, making that side negatively charged, and leaving the side closest to the ruler positively charged (that is, the bead is polarised). The force of attraction between the negative ruler and the positive side of the bead is stronger than the repulsion between the ruler and the negative side because the positive side is closer. So the bead is attracted.
10.4 The bead is repelled.
10.5 Some electrons are transferred from the ruler onto the bead, making the whole bead negatively charged. The ruler is still charged negatively. The two negatively charged objects repel.
QUESTION 11
11.1 energy transferred per coulomb of charge
11.2 3 V
11.3 3 A
11.4 Resistors in series divide the voltage of the battery in proportion to their resistances. The voltage across the parallel combination is one-third of the voltage across the 3 Ω resistor. Therefore, its resistance must also be one-third. Therefore 1 Ω.
11.5 2 Ω
11.6
- Q = IΔt
= (3) (120)
= 360 C
QUESTION 12
12.1
12.2 1 A
Paper 2
Data for Physical Sciences Grade 10 Chemistry (Paper 2)
Physical constants
Name | Symbol | Value |
Avogadro’s number | NA | 6,02 × 1023 |
Molar gas volume | 22,4 dm3.mol–1 | |
Standard temperature | 273 K (0 °C) | |
Standard pressure | 101,3 kPa |
Formulae
n = m
M
c = n
V
c = m
MV
SECTION A
QUESTION 1: ONE-WORD ANSWERS
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
1.1 When a liquid changes from a liquid into a gas. (1)
1.2 All water on and around the Earth. (1)
1.3 A chemical change that causes the surroundings to get cooler. (1)
1.4 The energy required to remove one electron from an atom of an element in the gaseous phase. (1)
1.5 The attraction that exists when two atoms share the same pair of electrons. (1) 5 × 1 = [5]
QUESTION 2: MATCHING PAIRS
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 property of a metal | A number of protons |
2.2 mass number | B number of electrons in the outer shell |
2.3 valency | C kilogram |
2.4 CaCO3 + heat → CaO + CO2 | D number of protons and neutrons |
2.5 SI unit for quantity of matter | E decomposition reaction |
F ductile | |
G mole | |
H brittle | |
I number of chemical bonds that an atom can form | |
J synthesis reaction |
5 × 1 = [5]
QUESTION 3: TRUE/FALSE
Indicate whether each of the following statements is true or false.
3.1 Elements in the same horizontal row in the periodic table have the same number of electrons in their outer energy levels.
3.2 Covalently bonded substances do not usually conduct electricity.
3.3 Oxidation is the process of water molecules surrounding ions when an ionic solid dissolves in water.
3.4 The chloride ion and the potassium ion have the same number of electrons.
3.5 Salt can become a conductor of electricity either by dissolving it in water or by melting it. 5 × 2 = [10]
QUESTION 4: MULTIPLE CHOICE
Choose the correct answer. Only write the letter of the answer that you select.
4.1 Atoms form bonds when valence electrons interact. When electrons are transferred from one atom to another the bond is:
- ionic.
- covalent.
- metallic.
- electronic. (3)
4.2 When sodium chloride dissolves in water, the . . . of the water molecule is attracted by the chlo- ride ion.
- hydrogen end, which is the positive pole
- hydrogen end, which is the negative pole
- oxygen end, which is the positive pole
- oxygen end, which is the negative pole (3)
4.3 This shows a representation of the nuclei of two atoms: ![]() . These atoms:
. These atoms:
- have the same mass numbers.
- are isotopes of the same element.
- have the same number of neutrons.
- have the same number of electrons. (3)
4.4 X represents some imaginary element with a fixed valency. The other elements below exhibit their normal valencies. Which one of the following formulae is wrong?
- XCl3
- X2S3
- X2O3
- X(NO3)2 (3)
4.5 Which set of coefficients will balance the following equation?
Al + H2O → Al2O3 + H2
- 2 : 1 : 3 : 3
- 2 : 3 : 1 : 3
- 3 : 2 : 1 : 3
- 2 : 3 : 3 : 1 (3) 3 × 5 = [15]
SECTION B
QUESTION 1
The following table shows the first ionisation energies for the elements of periods 1 and 2.
Period | Element | First ionisation energy (kJ.mol–1) |
1 | H | 1 312 |
He | 2 372 | |
2 | Li | 520 |
Be | 899 | |
B | 801 | |
C | 1 085 | |
N | 1 402 | |
O | 1 314 | |
F | 1 681 | |
Ne | 2 081 |
1.1 What is the meaning of the term ‘first ionisation energy’? (2)
1.2 Identify the general pattern of first ionisation energies in a period. (2)
1.3 Which two elements on the table above exert the strongest forces of attraction on their electrons? What property concerning the electrons in their outer shells is responsible for this? (4)
1.4 ‘All group 1 elements readily form positive ions’. Is this statement correct? Explain your answer by referring to the table. (3) [11]
QUESTION 2
Ernest Rutherford carried out one of the most important experiments in the history of science. He bombarded a very thin sheet of gold with fast-moving positively charged particles. He was able to trace the paths of the particles by seeing where they hit a spherical glass screen. From the results of his experiment, he was able to propose a new model for the structure of an atom.
2.1 Briefly describe the results of his experiment. (4)
2.2 Briefly describe the model of the atom that he was able to propose, based on these results. (4) 2 × 4 = [8]
QUESTION 3
A neutral atom has 20 electrons around its nucleus.
3.1 To which element does this atom belong? (2)
3.2 How many valence electrons does it have? (2)
3.3 How many energy levels does it have? (2)
3.4 Will this atom gain or lose electrons when it forms an ionic bond? Explain. (3)
3.5 How many electrons will this atom gain or lose during ionic bonding? (2)
3.6 After this atom has gained/lost electrons, which noble gas will have the same electron structure as the ion that is formed? (2) [13]
QUESTION 4
The element chlorine exists as three isotopes, one of which is 3517Cl.
4.1 What are ‘isotopes’ of an element? (2)
4.2 How many protons does the above isotope have? (2)
4.3 How many neutrons does it have? (2)
4.4 The other isotope of chlorine has 20 neutrons. Write its structure using the same notation as above. (2)
4.5 The atomic mass of chlorine is 35,5. What does this tell us about the ratio in which the two isotopes of chlorine occur in nature? (2) [10]
QUESTION 5
Potassium metal will burn in oxygen to form potassium oxide.
5.1 State Pauli’s exclusion principle. (2)
5.2 Use the ‘arrows in boxes’ notation to show the electron configuration of an oxygen atom. Label the energy levels and orbitals. (4)
5.3 Use Lewis structures to show the ionic bonding between potassium and oxygen atoms. (4)
5.4 Write the balanced chemical equation for the reaction between potassium and oxygen. (3) [13]
QUESTION 6
Sodium chloride is soluble in water.
6.1 Draw the Lewis structure for a water molecule. (3)
6.2 What is meant by the ‘electronegativity’ of an element? (2)
6.3 Instead of using a pair of dots or a dot and a cross to represent a shared pair of electrons in a covalent bond, a single line may be used. This method is called a Couper structure. Draw the Couper structure for a water molecule, showing the approximate bond angle in the structure. (2)
6.4 Explain why the water molecule is a dipole. Use the Couper structure sketch to illustrate your answer. (4)
6.5 Explain briefly the process that occurs when sodium chloride dissolves in water. Include in your explanation a sketch showing the positions of the water molecules around the ions. (7)
6.6 Write a chemical equation, including the phases, to represent this dissolution process. (3) [21]
QUESTION 7
You are given the following solubility rules:
- All nitrates are soluble.
- Salts of sodium, potassium and ammonium are soluble.
- All chlorides are soluble, except silver chloride and lead chloride.
- All sulphates are soluble, except the sulphates of calcium, barium, lead and silver.
- All carbonates are insoluble, except the carbonates of sodium, potassium and ammonium.
- All hydroxides are insoluble, except the hydroxides of sodium, potassium and ammonium.
Certain chemical reactions are classified as ‘precipitation reactions’.
7.1 What is a precipitate in this context? (2)
7.2 Is lead chloride soluble in water? (1)
7.3 You mix together solutions of sodium nitrate and potassium sulphate. Will a precipitate form? (Answer simply Yes or No.) (2)
7.4 You mix together solutions of silver nitrate and potassium chloride. A precipitate is formed.
7.4.1 Write down the name of the precipitate. (2)
7.4.2 Write a chemical equation for this reaction, including the states/phases. (4)
7.4.3 This is an example of a ‘precipitation reaction’. What other classification name applies to this reaction? (2) [13]
QUESTION 8
Write chemical formulae for the following compounds:
8.1 aluminium sulphate (2)
8.2 calcium hydroxide (2)
8.3 ammonium carbonate (2)
8.4 silver phosphate (2)
8.5 beryllium sulphide (2) 5 × 2 = [10]
QUESTION 9
Write balanced chemical equations for the following reactions:
9.1 hydrogen sulphate + sodium carbonate → sodium sulphate + carbon dioxide + water (5)
9.2 potassium + hydrogen oxide (water) → potassium hydroxide + hydrogen (5) [10]
QUESTION 10
Balance the following equations:
10.1 P + Cl2 → PCl3 (2)
10.2 Mg2SiO4 + H2O → Mg(OH)2 + H4SiO4 (2) [4]
QUESTION 11
Copper (II) sulphate (CuSO4) is formed when copper (II) oxide reacts with sulphuric acid. Water is the other product in this reaction. (Express answers correct to two decimal places.)
11.1 Write a balanced chemical equation for the reaction between copper (II) oxide and sulphuric acid. (4)
11.2 Calculate the percentage copper in copper (II) sulphate. (5)
11.3 Calculate the mass of copper (II) sulphate that must be dissolved in water to make up 250 cm3 of solution of concentration 0,4 mol.dm–3 (5) [14]
QUESTION 12
The empirical formula of a compound is found to be HO
12.1 What is meant by the ‘empirical formula’ of a compound? (2)
12.2 The formula mass of this compound is 34. What is the molecular formula of this compound? (2)
12.3 A compound was analysed by a chemist, who found that the compound contained 31,8 g potas- sium, 29,0 g chlorine and 39,2 g oxygen. Calculate the empirical formula of the compound. (6) [10]
QUESTION 13
You are given the following balanced chemical equation. (Express answers correct to 2 decimal places.)
CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
13.1 Calculate the mass of CaCl2 that will be obtained if 17 g of CaCO3 is reacted completely with HCl. (5)
13.2 Calculate the volume of CO2, measured at STP, that will be obtained. (5)
13.3 Why is it necessary for the volume of the gas to be measured at a particular temperature and pressure? (2) [10]
QUESTION 14
For this question, you may refer to the solubility rules given in question 7. You have a solution of barium chloride in a reagent bottle. You are given three test tubes: A, B and C. They contain the following solutions:
- copper sulphate
- sodium nitrate
- potassium carbonate
You add some barium chloride solution to each test tube.
14.1 In which two test tubes will you see a precipitate? (2)
14.2 Name the precipitates formed. (4)
14.3 What will you see if a solution of nitric acid is added to each test tube containing precipitates? (3)
14.4 Write an equation for the reaction described in 14.3. (3) [12]
QUESTION 15
15.1 Ammonia is a gas that has a very strong smell that actually hurts your nose and throat a bit and makes your eyes water. In terms of the kinetic molecular theory, explain why diffusion takes place when ammonia gas is released in a classroom and eventually every member of the class can smell the gas. (3)
15.2 What is meant by ‘sublimation’? Give an example of a substance that sublimes. (3) [6]
Total marks: 200
Answers
SECTION A
QUESTION 1: ONE-WORD ANSWERS
1.1 evaporates (or boils)
1.2 hydrosphere
1.3 endothermic
1.4 ionisation energy
1.5 covalent bond
QUESTION 2: MATCHING PAIRS
2.1 F
2.2 D
2.3 I
2.4 E
2.5 G
QUESTION 3: TRUE/FALSE
3.1 False
3.2 True
3.3 False
3.4 True
3.5 True
QUESTION 4: MULTIPLE CHOICE
4.1 A
4.2 A
4.3 C
4.4 D
4.5 B
SECTION B
QUESTION 1
1.1 First ionisation energy is the amount of energy required to remove the outermost (or first) electron from an atom in the gaseous phase.
1.2 Increases
1.3 He and Ne . They both have full outer shells.
1.4 No. A lot of energy is required to remove an electron from H, so H usually forms covalent bonds.
QUESTION 2
2.1 While nearly all the charged particles went straight through the gold, some were deviated from their path and a very small number were even reflected straight back.
2.2 The fact that nearly all went straight through meant that most of the atom must be empty space. Deviation was caused by repulsion of positive charge, so the positive charge and most of the mass of the atom is concentrated in a very small space, the nucleus. The electrons surround the nucleus to make up the volume of the atom.
QUESTION 3
3.1 Ca
3.2 3
3.3 3
3.4 Lose electrons. It is a metal, which has a weak attraction for outer shell electrons.
3.5 2
3.6 Ar
QUESTION 4
4.1 Isotopes are atoms of the same element that have the same atomic number but different mass number (or the same number of protons, but a different number of neutrons).
4.2 17
4.3 18
4.4 3717 Cl
4.5 The ratio of Cl–35 to Cl–37 must be approximately 1:3.
QUESTION 5
5.1 The maximum number of electrons that an orbital can contain is 2.
5.2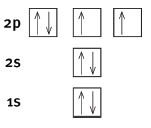
- Correct arrows and boxes
- Correct labelling
5.3![]()
- Correct reactants
- Correct product
5.4 2 K + O2 → K2O
QUESTION 6
6.1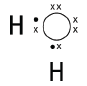
6.2 Electronegativity is the force of attraction that an atom has on a shared pair of electrons in a covalent bond.
6.3 Show bond angle > 90° 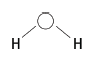
6.4 The electronegativity of O is greater than that of H. So, the shared pair of electrons is closer to the O than the H atoms, making the O end slightly negative and the H ends slightly positive. Couper structure 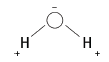
6.5 The O end of water molecules attract the H+ ions, while the H ends attract the Cl– ions. This causes the NaCl crystals to dissociate (or separate) into Na+ and Cl– ions. The water molecules now surround the ions, as in the sketch. This process is called hydration. Sketch 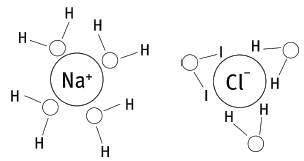
6.6 NaCl(s) → Na+(aq) + Cl–(aq)
QUESTION 7
7.1 A solid, insoluble substance
7.2 No
7.3 No
7.4.1 Silver chloride
7.4.2 AgNO3(aq) + KCl(aq) → AgCl(s) + KNO3(aq)
7.4.3 Ion exchange
QUESTION 8
8.1 Al2(SO4)3
8.2 Ca(OH)2
8.3 (NH4)2CO3
8.4 Ag3PO4
8.5 BeS
QUESTION 9
9.1 H2SO4 + Na2CO3 → Na2SO4 + CO2 + H2O
9.2 2 K + 2 H2O → 2 KOH + H2 (Correct balance )
QUESTION 10
10.1 2 P + 3 Cl2 → 2 PCl3
10.2 Mg2SiO4 + 4 H2O → 2 Mg(OH)2 + H4SiO4
QUESTION 11
11.1 CuO + H2SO4 → CuSO4 + H2O
11.2 Formula mass
- CuSO4 = 63,5 + 32 + 64 = 159,5
% Cu - 63,5 ×100
159,5
= 40,81%
11.3
- c = m m - MV - 159,5 x 0,25
MV c 0,4
= 99,70 g
QUESTION 12
12.1 The empirical formula is the simplest ratio of the atoms in the molecule or formula unit.
12.2 H2O2
12.3 In 100 g of the compound, we have 31,8 g K; 29,0 g Cl; 39,2 g O.
- Using n = M we have 31,8 mol K ; 29,0 mol Cl ; 39,2 mol O
M 39 35,5 16
= 0,82 mol K; 0,82 mol Cl; 2,45 mol O
Ratio = 1 : 1 : 3
Empirical formula = KClO3
QUESTION 13
13.1
- CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
1 mol CaCO3 forms 1 mol Ca Cl2
100 g Ca CO3 forms 111 g Ca Cl2
17 g CaCO3 forms x g CaCl2
x = 17 × 111 = 18,87 g
100
13.2
- 1 mol CaCO3 forms 22,4 dm3 CO2 at STP
100 g CaCO3 forms 22,4 dm3 CO2 at STP
17 g CaCO3 forms x dm3 CO2 at STP
x = 17 × 22,4 = 3,81 dm3
100
13.3 Volume increases as temperature increases and decreases as pressure decreases.
QUESTION 14
14.1 A and C
14.2 Barium sulphate and barium carbonate
14.3
- Barium sulphate precipitate is not affected.
Barium carbonate precipitate disappears and bubbles of gas are seen.
14.4 CaCO3 + 2 HNO3 → Ca(NO3)2 + CO2 + H2O [12]
QUESTION 15
15.1 Gas particles are moving at high speed in all directions, colliding with one another and any- thing in their path. There are very big spaces between the particles of air, so the ammonia molecules move through these spaces until they are evenly spread in the classroom.
15.2 Sublimation is the change from solid to gas without going through the liquid phase. Examples include solid CO2 (dry ice), naphthalene, and so forth.
Chemical Systems Questions and Answers Grade 10
Questions
Question 1: Multiple choice
Choose the correct answer. Only write the letter of the answer you select.
1.1 The interchange of water between Earth’s surface and the atmosphere is called:
- transpiration.
- precipitation.
- the water cycle.
- evaporation. (3)
1.2 The system consisting of all living things is the:
- hydrosphere.
- biosphere.
- atmosphere.
- lithosphere. (3)
1.3 What provides the energy to allow for a continuous water cycle?
- the Sun
- the Earth
- wind
- the hydrosphere (3)
1.4 The term ‘hydrosphere’ describes one of the Earth’s spheres and it includes all:
- of the water above and below the continents only.
- of the water on the planet.
- the fresh water on the planet only.
- of the liquid water on the Earth, minus ice. (3)
1.5 Which process does not produce carbon dioxide?
- burning of coal
- respiration (breathing)
- running of motor car engines
- transpiration (3) [15]
Question 2: One-word answers
Name the four interconnected systems that make life on Earth possible. (4) [4]
Question 3: Long question
Describe one useful way and one harmful way in which the hydrosphere interacts with:
3.1 the lithosphere. (4)
3.2 the biosphere. (4)
3.3 the atmosphere. (4) [12]
Question 4: Long question
Describe the ways in which water escapes from the ocean and is eventually returned to the ocean. (6) [6]
Question 5: Long question
Describe possible causes of pollution in rivers and dams. (6) [6]
Question 6: Long question
Describe two advantages and two disadvantages of building large dams. (8) [8]
Answers
Question 1: Multiple choice
1.1 C
1.2 B
1.3 A
1.4 B
1.5 D
Question 2: One-word answers
- hydrosphere
- atmosphere
- lithosphere
- biosphere
Question 3: Long question
3.1 Water enters the lithosphere through rain, providing an underground water supply (aquifers). Water dissolves nutrients in the soil that enable plants to grow,
in turn providing food for animals. Water runs off the land into rivers and dams. Water also dissolves harmful chemicals that can pollute rivers and dams.
Heavy rain can cause soil erosion.
3.2 Plants and animals cannot survive without water, as it is the solvent in which all the chemical reactions of living processes take place. Water dissolves nutrients in the soil that enable plants and in turn animals to grow. Heavy rain can cause flooding, destroying crops and causing the death of animals and humans. Soil erosion, caused by heavy rain, can make soil less fertile.
3.3 Water on Earth evaporates, providing water vapour in the atmosphere that can precipitate, providing water for the biosphere. The oxides of carbon, sulphur
and nitrogen are gases given off during the burning of fossil fuels. They dissolve in the water vapour in the atmosphere, causing acid rain.
Question 4: Long question
Ocean water evaporates forming water vapour in the atmosphere. Drops of water collect and can precipitate directly back into the ocean. Precipitation on land can run off to form rivers that flow back into the ocean. Water that collects underground can flow underground into the ocean.
Question 5: Long question
Acid rain caused by gases such as CO2, SO2, N2O and NO2 dissolving in water vapour in the air. Fertilisers and other chemicals dissolving in rain water and running into rivers and dams. Chemicals from industry being discharged straight into rivers and dams.
Question 6: Long question
- Advantages of large dams: A large amount of water can be retained for irrigation and can be provided for use by people and animals in times of drought. The water could be used in hydro-electric schemes (to generate electricity).
- Disadvantages: Areas below the dam may experience drought, flooding may occur when floodgates/sluices are opened. The environment is altered and
people and animals may be displaced.
Chemical Systems - Physical Science Grade 10 Study Guide
Overview
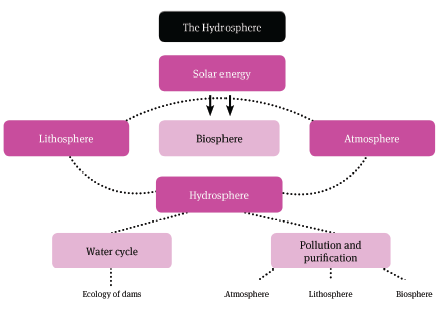
Summary
1 Interconnected systems
1.1 Interconnected systems and the water cycle
- There are four interconnected systems that make life on Earth possible. These are:
- atmosphere – the layer of gas surrounding the Earth, mostly nitrogen, oxygen and carbon dioxide
- lithosphere – the solid, outermost part of the Earth’s crust
- hydrosphere – all the Earth’s water
- biosphere – all living things (plants and animals) on Earth.
- Water moves through these spheres, dissolving chemicals and promoting growth of plants and animals. It also has the effect of moderating the temperature of the Earth – not allowing it to get too hot during the day or too cold during the night.
- The water cycle describes the changes water undergoes as it circulates through the different spheres. Important processes in the cycle are:
- evaporation – liquid water turns to water vapour
- precipitation – the condensation of water vapour to form liquid water (rain, dew)
- interception – the taking up of water by plants and animals
- transpiration – the giving off of water through the leaves of plants
- infiltration – the trickle down of water into the lithosphere to form ground water.
1.2 Pollution and purification
While water dissolves beneficial nutrients that are taken up by plants and animals, it also dissolves chemicals that are harmful.
- In the atmosphere, carbon dioxide and the oxides of sulphur and nitrogen are gases emitted by the burning of fossil fuels that pollute the atmosphere. They dissolve in the water vapour, resulting in acid rain.
- In the lithosphere, water dissolves fertilisers and pesticides, which pollute rivers and ground water. These can promote abnormal plant growth in rivers and dams, causing the depletion of oxygen. This can cause aquatic animals and organisms to die.
- In the biosphere, water can carry harmful bacteria from decaying and infected plants and animals into ground water. This can cause sickness in animals and humans.
Water can be purified by physical means (filtration), biological means (bacteria that remove waste products) and chemical means (mainly chlorination).
1.3 The ecology of dams
Dams are constructions that limit the flow of water in a river or stream. They are beneficial in providing water during drought and limiting flooding during heavy rain. They can provide hydro-electricity and boating and other leisure activities.
However, they also have an adverse effect on the ecology of the surrounding areas. Large areas are flooded, humans and animals are displaced and there is less provision of water downstream of the dam. Engineers have to strike a balance between environmental concerns, sustainability and economic and agricultural needs.
Mechanics Questions and Answers Grade 10
Questions
Question 1: Multiple choice
Choose the correct answer. Only write the letter of the answer you select.
1.1 When vectors X and Y are added, the resultant R is incorrectly represented in dia- gram: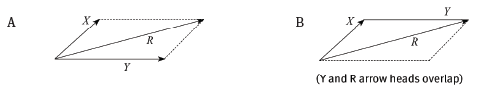

(3)
1.2 An athlete completed one lap of a 400 m oval track in 60 s. The magnitude of her average velocity for the race was:
- 6,67 m.s–1.
- 15 m.s–1.
- 0 m.s–1.
- 4,8 m.s–1. (3)
1.3 A car starts from rest and travels in a straight line for 10 s with a uniform acceleration of 2 m.s–2. From the end of one second to the end of the next second, the car goes:
- 2 m further than in the previous second.
- 3 m further than in the previous second.
- 6 m further than in the previous second.
- 9 m further than in the previous second. (3)
1.4 A trolley moves to the right with increasing velocity and uniform acceleration. The ticker tape that it pulls through a ticker timer looks like this: (3)
(3)
1.5
The velocity–time graph that corresponds with the above position–time graph is:
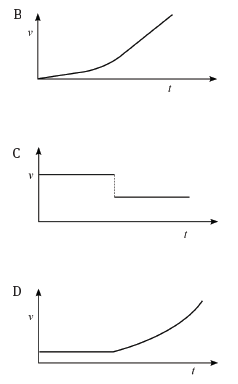 (3)
(3)
1.6
The position–time graph that matches the above velocity–time graph is:
 (3)
(3)
1.7
The acceleration–time graph that corresponds with the above velocity–time graph is: 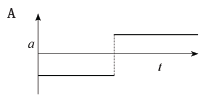
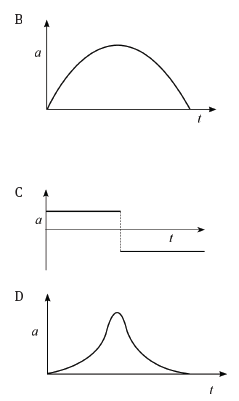 (3)
(3)
1.8 When a car’s speed doubles:
- the stopping distance is about twice as long.
- the thinking distance is about four times as long.
- the braking distance is about four times as long.
- the stopping distance is about four times as long.
1.9 An object with potential energy EP is a height h above the Earth’s surface. It is then raised to a height 2 h. The new potential energy is:
- 3 EP.
2 - 2 EP.
- 3 EP.
- 4 EP. (3)
1.10 A stone falls through the air from a high bridge. As it falls:
- mechanical energy is conserved.
- kinetic energy is conserved.
- potential energy is conserved.
- potential energy is transferred to kinetic energy and heat. (3)
Question 2: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
2.1 Two force vectors, drawn to scale, are equal if they have the same length. (2)
2.2 Distance is change in position; it is a straight-line from the original position to the final position. (2)
2.3 Change in velocity is indicated by the area under a position–time graph. (2)
2.4 Acceleration is change in velocity. (2)
2.5 When a moving object doubles its speed, its kinetic energy also doubles. (2)
Question 3: One-word answers
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
3.1 A physical quantity that has magnitude but not direction. (1)
3.2 The rate of change of displacement. (1)
3.3 Velocity at a particular time. (1)
3.4 Area under a velocity–time graph. (1)
3.5 When an object falls in the absence of air resistance, mechanical energy is … (1)
Question 4: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
4.1 force | A 90 km.h–1 |
4.2 area under an acceleration–time graph | B kinetic energy (EK) |
4.3 mechanical energy (EM) | C m |
4.4 acceleration | D mass |
4.5 25 m.s–1 | E change in velocity |
4.6 energy of motion | F m |
4.7 area under a velocity–time graph | G potential energy (EP) |
4.8 energy of position | H m |
4.9 velocity | I EK + E |
4.10 kilogram | J weight |
K 72 km.h–1 | |
L always conserved | |
M change in position |
[10]
Question 5: Long questions
Consider the physical quantities mass, weight, distance, displacement, speed, velocity, acceleration and energy.
5.1 Give the name and the abbreviation of the unit (or units) in which each is meas- ured. (6)
5.2 List those that are vector quantities and those that are scalar quantities. (4)
5.3 Using a scale of 10 mm to represent 10 m, draw vectors to represent:
5.3.1 a displacement of 50 m north. (2)
5.3.2 a displacement of 80 m east. (2)
5.4 Find the resultant of the two displacements in question 5 3 graphically:
5.4.1 by the tail-to-head method. (4)
5.4.2 by the tail-to-tail method. (3)
5.5 Check your answers to question 5.4 by calculating the resultant. Supply a rough sketch that helps to explain your calculation. (9) [30]
Question 6: Long questions
6.1 A golf ball falls vertically from the roof of a 12 m high building. It bounces upwards to a height of 9 m above the ground. Take the upward direction as the positive direction. What is the ball’s displacement, (1) when it strikes the ground; (2i) at the highest point of the bounce:
6.1.1 relative to the ground. (2)
6.1.2 relative to the top of the building. (2)
6.2 A cyclist rides halfway around a circular track with a radius of 140 m. She starts facing north and rides clockwise around the track. What is:
6.2.1 the distance that she rides? (3)
6.2.2 her displacement? (2) [9]
Question 7: Long questions
7.1 A 707 km road journey from Durban to Johannesburg took 7 hours. Calculate the average speed for the trip:
7.1.1 in km.h–1. (4)
7.1.2 in m.s–1. (3)
7.2 If the displacement from the starting point in Durban to the ending point in Johannesburg was 644 km, calculate the magnitude of the average velocity for the trip (in km.h–1). (4)
7.3 A skier, first moving at 8 m.s–1 in a straight line on level snow, accelerates down a slope. After 10 s on the slope, she reaches a velocity of 20 m.s–1. Calculate her average acceleration. (6) [17]
Question 8: Long questions
A train transporting iron-ore from Sishen to Saldahna Bay travels on a straight track at 40 km.h–1 for 4 hours. For this part of the trip, plot:
8.1 a position–time graph. (5)
8.2 a velocity–time graph. (5)
8.3 A ski-jumper starts from rest and moves down the first part of a straight track with constant acceleration. Taking the direction of motion as the positive direction, show in sketch graphs:
8.3.1 the shape of the position–time graph. (3)
8.3.2 the shape of the velocity–time graph. (3)
8.3.3 the shape of the acceleration–time graph. (3) [19]
Question 9: Long questions
The velocity–time graph of an object is shown below.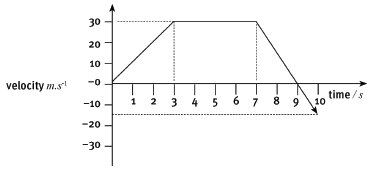
9.1 Describe the motion:
9.1.1 during the first 3 s. (3)
9.1.2 from t = 3 s until t = 7 s. (2)
9.1.3 from t = 7 s until t = 9 s. (3)
9.1.4 from t = 9 s until t = 10 s. (2)
9.2 Use the graph to find:
9.2.1 the uniform acceleration during the first 3 s of the motion. (5)
9.2.2 the uniform acceleration during the last 3 s of the motion. (4)
9.2.3 the displacement during the first 3 s of the motion. (5)
9.2.4 the displacement of the body while it moved with constant velocity. (3)
9.2.5 the displacement during the last 3 s of the motion. (5)
9.2.6 the total displacement for the full trip. (2)
9.2.7 the average velocity for the full trip. (5) [39]
Question 10: Long questions
The equations of uniform motion make use of the following symbols:
∆x, ∆t, vi , vf and a.
10.1 What quantity does each symbol represent? (5)
10.2 Name the abbreviated SI unit(s) in which each quantity is measured. (4)
10.3 An object moves horizontally in a straight line from rest with a constant acceleration of 2 m.s–2. Complete the table below by replacing the question numbers with the correct values. (Note: You should only find it necessary to calculate in one of the rows.)
After a time of exactly: | 1 s | 2 s | 3 s | 4 s |
Velocity of object | 10.3.1 (1) | 10.3.2 (1) | 10.3.3 (1) | 10.3.4 (1) |
Acceleration of object | 10.3.5 (1) | |||
Displacement from starting position | 10.3.6 (3) | 10.3.7 (3) | 10.3.8 (3) | 10.3.9 (3) |
[23]
Question 11: Long questions
11.1 A motor cyclist travels at 18 m.s–1 along a straight road. He accelerates uniformly for 6 s to a velocity of 30 m.s–1. Calculate:
11.1.1 the acceleration of the motor cycle. (6)
11.1.2 the displacement of the motor cycle while accelerating. (6)
11.2 A car moving at 12 m.s–1 along a straight road accelerates at 2 m.s–2 for 5 s. Calculate:
11.2.1 the car’s displacement after 5 s. (6)
11.2.2 the car’s velocity after 5 s. (6)
11.3 A car moving with a velocity of 15 m.s–1 is brought to rest over a distance of 37,5 m when the brakes are applied. Calculate the magnitude of the acceleration. (6)
11.4 A bullet leaves the barrel of a rifle at 1000 m.s–1. If the barrel is 1 m long, calculate the magnitude of the acceleration of the bullet. Assume that the acceleration of the bullet is constant. (5)
11.5 A car travelling at 20 m.s–1 has its brakes applied and slows down at a constant rate of 10 m.s–2. What is the stopping distance of the car? (5) [40]
Question 12: Long questions
12.1 A man with a mass of 75 kg climbs a ladder to the flat roof of his house. If the vertical height of the roof is 3 m above the ground, how much gravitational potential energy has he gained? (5)
12.2 What is the gravitational potential energy of an object of mass 40 kg that is at a height of 30 m above the ground? (5)
12.3 Calculate the kinetic energy of a 72 kg parachutist, falling with a speed of 30 m.s–1. (4)
12.4 An aircraft of mass 4000 kg flies with a speed of 150 m.s–1 and maintains an altitude of 5000 m. Calculate its mechanical energy relative to the Earth’s surface. (7)
12.5 An object of mass 500 kg is raised to a height of 40 m. If the object falls freely from this height, what will its velocity be just before it strikes the ground? (6)
[27]
Answers
1.1 C
1.2 A
1.3 B
1.4 A
1.5 A
1.6 C
1.7 C
1.8 C
1.9 B
1.10 D
2.1 Two force vectors, drawn to scale, are equal if they have the same length and direction.
2.2 Displacement is change in position; it is a straight-line from the original position to the final position.
2.3 Change in position is indicated by the area under a velocity–time graph.
2.4 Acceleration is the rate of change in velocity.
2.5 When a moving object doubles its speed, its kinetic energy becomes four times greater.
3.1 scalar
3.2 velocity
3.3 instantaneous velocity
3.4 displacement
3.5 conserved
4.1 J
4.2 E
4.3 I
4.4 H
4.5 A
4.6 B
4.7 M
4.8 G
4.9 C
4.10 D
Question 5: Long questions
5.1
- mass: kilogram (kg)
- weight: newton (N)
- distance and displacement: metre (m)
- speed and velocity: metre per second (m.s–1) ?
- acceleration: metre per second squared (m.s–2) ?
- energy: joule (J) ?
5.2 vectors: weight, displacement, velocity, acceleration ??
scalars: mass, distance, speed, energy ??
5.3.1 & 5.3.2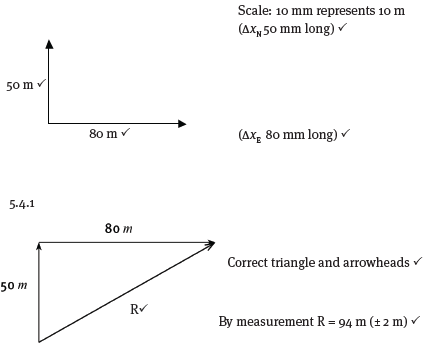
In the direction 58º (± 1º) east of north ?
5.4.2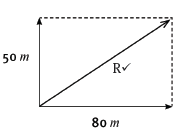
Correct rectangle and arrowheads ?
By measurement R = 94 m (± 2 m)
In the direction 58º (± 1º) east of north ?
5.5 By Pythagoras:
labelled sketch diagram ??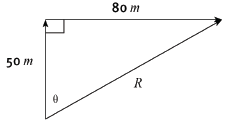
R2 = 802 + 502
∴R = √8900
= 94,34m
tan θ = 80
50
∴θ =58º
By calculation, the resultant R = 94,34 m in the direction 58º east of north ?
Question 6: Long questions
6.1.1
- (1) 0 ?
(2) +9 m ?
6.1.2
- (1) –12 m ?
(2) –3 m ?
6.2.1
- Circumference of circle = 2∏r
Half circumference of circle = ∏r? =2∆r 22 × 140 = 440m
7
Distance = 440 m ?
6.2.2
- Displacement = 140 m ? east ?
Question 7: Long questions
7.1.1
- average speed = D ?= 707 ??=101 km.h–1?
∆t 7
7.1.2
- 101 km = 101000 m ??= 28,06 m.s–1?
h 3600 s
7.2
- magnitude of average velocity
= ∆x = 644 ?= ??= 92 km.h–1?
∆t 7
7.3 Average acceleration
- = ∆v = vf − vi = ? = (20–8) ???= 1,2 m.s–2 ?
∆t ∆t 10
The acceleration has a magnitude of 1,2 m.s–2 and is in the same direction as the skier’s motion.?
Question 8: Long questions
8.1
axes ? values on axes ??
shape of graph ??
8.2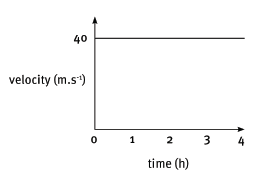
axes ? values on axes ??
shape of graph ??
8.3.1
axes ?
shape of graph ??
8.3.2
axes ?
shape of graph ??
8.3.3
axes ?
shape of graph ??
Question 9: Long questions
9.1.1 Uniform acceleration ? from rest to 30 m.s–1 ? in the positive direction ?
9.1.2 Constant velocity ? of 30 m.s–1 in the positive direction ?
9.1.3 Uniform negative acceleration ? from 30 m.s–1 in the positive direction to rest ?
9.1.4 Uniform negative acceleration ? from rest to 15 m.s–1 ? in the negative direction ?
9.2.1 acceleration = gradient
- = (30 − 0) ??= 10 m.s–2 ?
(3 − 0)
in the direction of motion ?
9.2.2 acceleration = gradient ?
- = ∆x
∆y
= (−15 − 30) ??= –45 − 15 m.s–2 ?
(10 − 7) 3
9.2.3 displacement = triangular area under graph = ½ bh ?
= ½ × 3 ? × 30 ?
= 45 m ? in the direction of motion ?
9.2.4 displacement = rectangular area under graph = l × b
= 4 ? × 30 ?
= 120 m ? in the direction of motion
9.2.5 displacement = sum of two triangular areas under graph (area = ½ bh)
= (½ × 2 ? × 30 ?) + [½ × 1 ? × (–10) ?]
= 30 – 5
= 25 m ? in the direction of motion
9.2.6 total displacement = total area under graph
= 45 + 120 + 25 ?
= 190 m ? in the direction of motion
9.2.7 average velocity = ∆x ?= 190 ??= 17,27 m.s–2 ?in the direction of motion ?
∆y 11
Question 10: Long questions
10.1
- ∆x displacement (change in position)?
- ∆t change in time ?
- vi initial velocity ?
- vf final velocity ?
- a uniform acceleration ?
10.2
- Dx m ?
- ∆t s ?
- vi and vf m.s–1 ?
- a m.s–2 ?
10.3
After a time of exactly: | 1 s | 2 s | 3 s | 4 s |
Velocity of object | 2 m.s–1 ? | 4 m.s–1 ? | 6 m.s–1 ? | 8 m.s–1 ? |
Acceleration of object | all 2 m.s–2 ? | |||
Displacement from starting position | 1 m ??? | 4 m ?? | 9 m ?? | 16 m ?? |
10.3.6 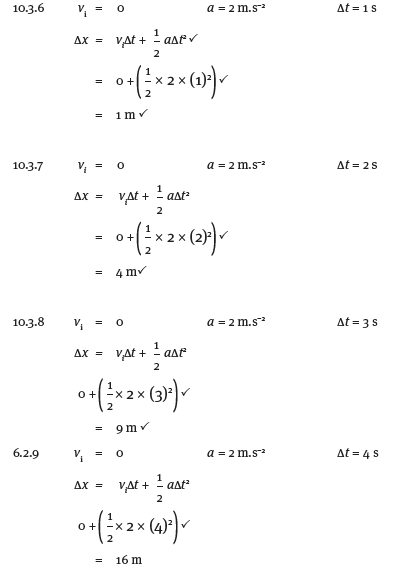
Question 11. Long questions
11.1.1 vi = 18 m.s–1 vf = 30 m.s–1 00100 ∆t = 6 s
Average acceleration = ∆v = vf − vi ?= (30 − 18) ???= 2 m.s–2 ?
∆t ∆t 6
in the direction of motion. ?
11.1.2
- ∆x = (vf + vi) ∆t?
2
= (18? + 30 ?) 6
2
= 144 m in the direction of motion?
11.2.1= 85 m in the direction of motion?
11.2.2 vf = v1 + a∆t ?
= 12? + (2 × 5)??
= 22 m.s–1 in the direction of motion?
11.3 ∴ a = −3 m.s–2 ?
The magnitude of the acceleration is 3 m.s–2, ?and the acceleration is negative.
11.4 ∴ a = 5 × 105 m.s –2 ?
11.5 ∴ ∆x = 20 m
Question 12: Long questions
12.1
- m = 75 kg g = 9,8 m.s–2 h = 3 m
EP = mgh ? = 75 ? × 9,8 ? × 3 ? = 2205 J ?
12.2
- m = 40 kg g = 9,8 m.s–2 h = 30 m
EP = mgh ? = 40 ? × 9,8 ? × 30 ? = 11 760 J ?
12.3
- m = 72 kg v = 30 m.s–1
EK = ½ mv2 ?= 0,5 × 72 ? × (30)2 ?= 32 400 J ?
12.4
- m = 4000 kg v = 150 m.s–1 g = 9,8 m.s–2 h = 5000 m
EM = EK + EP ?
= ½ mv2+ mgh ?
= [0,5 × 4000 ? × (150)2 ?] + (4000 x 9,8 ? × 5000 ?)
= (4,5 × 107) + (1,96 × 107)
= 6,46 × 107 J ?
12.5
- m = 500 kg g = 9,8 m.s–2 h = 40 m Because mechanical energy is conserved:
Ek + Ep1 (just above ground level) + Ek2 + Ep2 (at highest point)
1 mv2 + mgh (just above ground level) = 1 mv2 + mgh (at highest point)
2 2
1 mv2 + 0 (just above ground level) = 0 + mgh (at highest point)
2
Mechanics - Physical Science Grade 10 Study Guide
Overview
Summary
1 Motion and forces
Mechanics is the branch of physics that focuses on the motion of objects and the forces that act on them. Not all physical quantities can be treated in the same way. Two 2 kg masses lumped together give a bigger mass of 4 kg, but the combined effect of two forces acting on an object in different directions cannot be found by simple addition. It is important to learn how to work with physical quantities such as force before meeting similar difficulties in your study of motion.
When speaking of motion, many people use the words ‘velocity’ and ‘acceleration’ to describe the same thing, but this is a mistake. For example, it is quite common for a moving object to have a high velocity and very little or no acceleration. Getting correct ideas about concepts like this from the start makes things much easier later on. Some aspects of motion can be quite tricky to analyse. So you will be pleasantly surprised to find that straight-line motion at constant speed or with speed that changes by the same amount every second is relatively easy to describe in words, diagrams, graphs and equations.
The study of motion leads on to the energy transfers that cause all the excitement on a roller coaster. Our interest is in the energy possessed by an object because of its position above the ground and the energy it has when it is moving. We will study the interchange between these two energies in roller coasters and some other frictionless systems.
2 Vectors and scalars
- Scalar quantities (for instance, mass, charge, energy, time, distance and speed) have magnitude only.
- Vector quantities (for instance, force, weight, displacement, velocity and acceleration) have both magnitude and direction
- Vector quantities are represented by arrows called vectors.
- In a drawing to scale, the magnitude of a vector quantity is represented by the length of the vector and its direction is indicated by the arrowhead.
- Equal vectors have the same length and direction.
- Negative vectors act in the opposite direction to the chosen positive direction.
- Vectors can be added and subtracted and their magnitudes can be multiplied.
- A resultant vector is the single vector that has the same effect as a number of separate vectors acting together.
- Resultant vectors can be determined:
- graphically, using the tail-to-head and tail-to-tail methods
- by calculation.
3 Motion in one dimension
- Motion is measured relative to a frame of reference. A frame of reference has an origin (or reference point) and a set of directions.
- Motion in one dimension is straight-line motion in the forward or backward direction.
- Position is always stated relative to a reference point and may be positive or negative. For position, usually use the symbol x; if motion is vertical, use the symbol y.
- Displacement (∆x or ∆y) is change in position. It is a vector quantity: the straight-line distance from a starting position to a new position, together with the direction.
- Distance (D) is the length of the actual path taken between a starting position and a new position. It is a scalar quantity.
- Average speed (v) is a scalar quantity: the distance travelled divided by the time taken (∆t).
v = D
∆t - Average velocity is a vector quantity: the displacement divided by the time taken.
v = ∆x
∆t - Average acceleration (a) is the change in velocity divided by the time taken.
a = ∆v
∆t
3.1 Worked examples
- Sara runs a 200 m race in 30 s. Calculate her average speed.
Answer:
v = D
∆t
= 200
30
= 6,67 m.s–1 - Calculate Simo’s average velocity if it takes him 8 minutes to walk 400 m east and then 160 m west.
Answer:
v = ∆x
∆t
= 240
480
= 6,67 m.s∆1 east
The direction east is chosen as the positive direction.
∆t = 8 × 60 = 480 s ∆x = 400 – 160 = 240 m east - A cyclist moving at 12 m.s–1 stops pedalling at the bottom of a gentle slope and coasts to a stop. If this takes 5 s, calculate his acceleration.
Answer:
The initial direction of motion is chosen as the positive direction.
∆v = final velocity – initial velocity = (0 – 12) = –12 m.s–1
a = ∆v
∆t
= 12
5
= ∆2,4 m.s∆2, that is, 2,4 m.s–2 in the opposite direction to the initial motion
- Acceleration indicates how quickly the velocity is changing, but provides no information about the direction of motion.
- Acceleration is produced by a force, and always has the same direction as the force that is causing it.
- If we take the direction in which a car is facing as the positive direction, then: positive acceleration means there is a forward force acting, which will increase its velocity negative acceleration means there is an opposing force acting on it, which will either decrease its velocity (for instance, if the brakes are applied, or it coasts uphill) or increase its velocity backwards (if the car accelerates in reverse gear).
- Instantaneous velocity is the displacement divided by a very small time interval.
- Instantaneous speed is the magnitude of the instantaneous velocity.
4 Motion described in words, diagrams, graphs and equations
4.1 Motion with uniform velocity
- An object moving in a straight line with uniform velocity changes its position by the same amount in equal time intervals.
- A position–time graph for motion with uniform velocity is always a straight line with a positive or negative gradient.
- For motion in the positive direction, the graph has a positive gradient.

When motion is in the negative direction, the gradient is negative.
A position–time graph of a stationary object is horizontal.
The gradient of a position–time graph is equal to the uniform velocity.
A velocity–time graph for motion with uniform velocity is horizontal.
- The area under a velocity–time graph is equal to the displacement. The area under the graph is rectangular in shape. Motion is in the positive direction when the area under the graph is positive (above the horizontal time axis) and motion is in the negative direction when the area under the graph is negative (below the time axis).
4.2 Motion with uniform acceleration
- An object moving with uniform acceleration has a velocity that changes in magnitude by the same amount in equal time intervals.
- A position–time graph for motion with uniform acceleration is never a straight line. The gradient of the graph changes at a constant rate.
- For motion in the positive direction, the gradient of the graph increases steadily if the velocity is increasing.

- For motion in the positive direction, the gradient of the graph decreases steadily if the velocity is decreasing.

- The gradient of the tangent drawn to a position–time graph at a particular time is equal to the instantaneous velocity.

- A velocity–time graph for motion with uniform acceleration is always a straight line with a positive or negative gradient.
- For motion in the positive direction, the graph has a positive gradient if the velocity is increasing.

- For motion in the positive direction, the graph has a negative gradient if the velocity is decreasing.

- The gradient of a velocity–time graph is equal to the uniform acceleration.
- The area under a velocity–time graph is equal to the displacement. For motion with uniform acceleration, the area under the graph is triangular in shape. Motion is in the positive direction when the area under the graph is positive and motion is in the negative direction when the area under the graph is negative.

- An acceleration–time graph for motion with uniform acceleration is horizontal.
- The area under an acceleration–time graph is equal to the change in velocity. The area under the graph is rectangular in shape. For motion in the positive direction, the change in velocity is positive when the area under the graph is positive and the change in velocity is negative when the area under the graph is negative.

- Roads are safer when drivers understand the relationship between speed and stopping distance, when drivers are in full control of their faculties, when brakes are in good condition, when road conditions are favourable and when vehicles are fitted with safety features.
4.3 Kinematics equations
- Equations of uniform motion are used to solve problems involving motion in one dimension.
- The symbols used in the equations are:
- ∆x: displacement (or use ∆y it the change in position is vertical)
- ∆t: time a: unifom acceleration
vf : initial velocity vf : final velocity
- The equations are:

4.3.1 Worked examples
1 Jane cycles along a straight, level road at 4 m.s–1. Then she accelerates uniformly at 0,5 m.s–2 for 12 s. Calculate:
1.1 the final velocity
1.2 the displacement while she accelerates.
Answers:
Take the initial direction of motion as the positive direction.
1.1
- vi = 4 m.s–1 a = 0,5 m.s–2 ∆t = 12 s
vf = vi = a∆t2
= 4 + (0,5 x 12)
= 10m.s–1 in the direction of motion
1.2
- vi = 4 m.s–1 a = 0,5 m.s–2 ∆t = 12 s
∆x = v ∆t + 1 a∆t2
2
= (4 12) +{0,5 × 0,5 × (12)2}
2 Joe cycles along a straight, level road at 15 m.s–1. Then he stops pedalling and slows down uniformly over a displacement of 52 m. If his velocity falls by 1 m.s–1 every second, calculate:
2.1 the final velocity after 52 m
2.2 the time that he takes to cover the 52 m.
Answers:
Take the initial direction of motion as the positive direction.
2.1
- vi = 15 m.s–1 a = –1 m.s–2 ∆x = 52 m
v f2 = vt2 + 2a∆x
= (15)2 + {2 ×(–1) × 52}
= 225 – 104
∴ vf = √121
= 11m.s-1 in the direction of motion
2.2
- vi = 15 m.s–1 vf = 11 m.s–1 a = –1 m.s–2

5 Energy
- The gravitational potential energy of an object is the energy it has because of its position in the Earth’s gravitational field relative to a selected reference point.
- Gravitational potential energy is calculated using the formula:
EP = mgh - Kinetic energy is the energy possessed by an object as a result of its motion.
- Kinetic energy is calculated using the formula:
EK = ½ mv2 - Mechanical energy (EM) is the sum of gravitational potential energy and kinetic energy:
EM = EK + EP - Energy cannot be created or destroyed. It can only be changed from one form into another.
- In the absence of air resistance, the mechanical energy of an object moving in the Earth’s gravitational field is conserved.
- When energy transfers happen and mechanical energy is conserved, use the equation:
EK1 + EP1 = EK2 + EP2
5.1 Worked examples
1 A boy of mass 50 kg is ready to jump from a bridge into the water 4,9 m below. What is his potential energy:
1.1 relative to the bridge?
1.2 relative to the water surface?
Answers:
1.1 0
1.2
- m = 50 kg g = 9,8 m.s–2 h = 4,9 m
EP = mgh
= (50 × 9,8 × 4,9)
= 2401 J
2 After falling for 1 s, the boy in the previous example splashes into the water with a speed of 9,8 m.s–1. Calculate his kinetic energy as he strikes the water surface.
Answer:
- m = 50 kg v = 9,8 m.s–1
EK = ½ mv2
= [0,5 × 50 × (9,8)2]
= 2401 J
3 Calculate the mechanical energy of a sledgehammer of mass 3 kg moving downwards at 6 m.s–1 and 0,5 m above the ground.
Answer:
- m = 3 kg v = 6 m.s–1 g = 9,8 m.s–2 h = 0,5 m
EM = EK + EP
= ½ mv2 + mgh
= [0,5 × 3 × (6)2] + (3 × 9,8 × 0,5)
= 68,7 J
Chemical Change Questions and Answers Grade 10
QUESTIONS
Question 1: Multiple choice
Choose the correct answer. Only write the letter of the answer you select.
1.1 In the compound SO2, the ratio of the mass of sulphur to oxygen is always …
- 1:2.
- 2:1.
- 8:16.
- 1:1. (3)
1.2 The formula 3 Al2O3 represents …
- 3 atoms of aluminium and 6 atoms of oxygen.
- 3 atoms of aluminium and 2 atoms of oxygen.
- 3 formula units of Al2O3.
- 6 atoms of aluminium and 3 atoms of oxygen. (3)
1.3 You are given the unbalanced reaction Mg(OH)2 + HCl → MgCl2 + H2O The balanced reaction would be:
- 2 Mg(OH)2 + 2 HCl → 2 MgCl +2 H2O.
- Mg(OH) + 2 HCl → MgCl + 2 H2O.
- Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O.
- 2 Mg(OH)2 + 4 HCl → 2 MgCl2 + 4 H2O. (3)
1.4 In a physical change, which one of the following statements is true?
- The mass is conserved but energy is used up.
- The mass is not conserved but the energy is conserved.
- The number of molecules remains constant during the reaction.
- Only the number of atoms remains constant during the reaction. (3)
1.5 Which of the following will NOT cause a precipitate if added to a solution of silver nitrate?
- sodium chloride
- copper chloride
- magnesium sulphate
- magnesium nitrate (3)
1.6 The reaction that could be used to prepare a bromide salt is:
- copper oxide and sulphuric acid.
- silver nitrate and potassium bromide.
- ammonium chloride and potassium bromide.
- ammonium nitrate and potassium carbonate. (3)
1.7 An unknown salt forms a creamy yellow precipitate with silver nitrate. The precipitate does not dissolve in ammonium hydroxide solution. The salt probably is:
- potassium iodide.
- potassium nitrate.
- potassium bromide.
- potassium chloride. (3)
1.8 The ion-exchange reaction HNO3(aq) + NaOH(aq) → NaNO3(aq) + 2H2O(l) is an example of:
- an acid-base reaction.
- a gas-forming reaction.
- a redox reaction.
- a precipitation reaction. (3)
1.9 One mole of SO2 contains:
- 1 molecule.
- 6,02 × 1023 molecules.
- 3 atoms.
- 18,06 × 1023 molecules. (3)
1.10 The formula mass of C2O4H2.2H2O is:
- 110.
- 126.
- 90.
- 108. (3)
1.11 Which of the following has the greatest mass?
- 1 mole of Fe
- 0,5 mol FeCl2
- 0,1 mol FeSO4
- 0,2 mol Fe2O3 (3)
1.12 The number of moles of water molecules in 126 g of ice is:
- 18.
- 7.
- 7 × 6,02 × 1023.
- 6,02 × 1023. (3)
1.13 If you had 20 g each of the following compounds, which sample would have the largest number of moles?
- H2O
- NO2
- N2
- NH3 (3)
1.14 What is the number of atoms in 8 g of helium?
- 2 × 6,02 × 1023
- B 6,02 × 1023
- 4 × 6,02 × 1023
- 8 ÷ 6,02 × 1023 (3)
1.15 Which of the following does NOT represent one mole of hydrogen gas at STP?
- 2 g
- 22,4 dm3
- 6,02 × 1023 molecules
- 2 × 6,02 × 1023 molecules (3)
Question 2: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
2.1 The melting of ice is an example of a physical change. (2)
2.2 The mass ratio of carbon to oxygen in a molecule of CO2 will always be 1:2. (2)
2.3 All nitrates are soluble. (2)
2.4 The SI unit for measuring the quantity of a substance is kilogram. (2)
2.5 The number that is equal to one gram of a substance is called Avogadro’s number. (2)
Question 3: One-word answers
Provide the missing word or term in the sentences below. Write only the word or term next to the question number.
3.1 A positive ion is called a(n) … (1)
3.2 If solutions of barium chloride and magnesium sulphate are mixed, the solid that will form is … (1)
3.3 HO is the … formula of hydrogen peroxide. (1)
3.4 The state symbol for mercury at room temperature is … (1)
3.5 The souring of milk is an example of a … change. (1)
Question 4: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
4.1 an electrolyte | A mole |
4.2 mass ratio of 3:1 | B 6,02 × 1023 |
4.3 number of particles in 8 g of helium gas | C a physical change |
4.4 N2 + 3H2 → 2 NH3 | D CO |
4.5 mass ratio of 3:4 | E sucrose |
4.6 SI unit of quantity | F ammonium sulphate |
4.7 a non-electrolyte | G kilogram |
4.8 number of particles in 22,4 dm3 of helium gas at STP | H CH4 |
4.9 SI unit of mass | I a chemical change |
4.10 glass breaking | J 2 × 6,02 × 1023 |
[10]
Question 5: Long questions
5.1 Calculate the empirical formula of a substance that consists of 30,4% nitrogen, and 69,6% oxygen by mass. (7)
5.2 If the formula mass of the substance in 5.1 is 92, what is the molecular formula of the substance? (3) [10]
Question 6: Long questions
6.1. If a gas is collected at STP, under what conditions of temperature and pressure is it collected? (2)
6.2 A sample of calcium carbonate (CaCO3) was reacted with HCl in a closed contain- er according to the following equation:
CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
The carbon dioxide gas produced was collected at STP and occupied a volume of 33,6 dm3. Calculate the mass of calcium carbonate that reacted with the HCl.(7) [9]
Question 7: Long questions
7.1 Calculate the mass of potassium sulphate produced by 10 g of potassium hydrox- ide according to the equation:
2 KOH + H2SO4 → K2SO4 + 2 H2O (7)
7.2 What volume of hydrogen gas, measured at STP, would be produced when 4 g of magnesium reacts completely with excess H2SO4 according to the equation:
Mg + H2SO4 → MgSO4 + H2 (6) [13]
Question 8: Long questions
Aluminium oxide (Al2O3) is the starting compound for making high-temperature- resistant ceramic products.
8.1 What is the percentage of aluminium in aluminium oxide? (4)
8.2 How much aluminium sulphate Al2(SO4)3 is required to make up 250 ml of a 0,1 mol.dm-3 solution of this compound? (6) [10]
Question 9: Long questions
9.1 What is a precipitate? (2)
9.2 Complete the following ion-exchange reactions:
9.2.1 KCl(aq) + NaNO3(aq) → (2)
9.2.2 CaSO4(aq) + MgCO3(aq) → (2)
9.2.3 BaCl2(aq) + Na2SO4(aq) → (2)
9.2.4 K2SO4(aq) + Pb(NO3)2(aq) → (2)
9.3 For each reaction in 9.2, state if any precipitate is formed. If a precipitate is formed, give its formula. (4) [14]
Question 10: Long questions
10.1 You are given three unlabelled test tubes. One contains sodium bromide, one contains potassium iodide and one contains magnesium carbonate. List the steps you would take to identify what compound was in each sample. (8)
10.2 Give a balanced equation for the reaction that helped you to identify which sam- ple contained magnesium carbonate. (3) [11]
Question 11: Long questions
11.1 If 4,5 g of calcium burns in oxygen to form calcium oxide (CaO), what mass of CaO will form from that amount of calcium? (5)
11.2 Balance the following chemical reactions:
11.2.1 CH4 + O2 → CO2 + H2O (2)
11.2.2 Al + Cl2 → AlCl3 (2)
11.2.3 CO2 + H2O → C6H12O6 + O2 (2) [6]
Question 12: Long questions
The substance adenosine triphosphate (ATP) is important because it provides a way of transferring energy in living cells.
12.1 A sample of 1,6270 g of ATP was analysed and found to contain 0,3853 g carbon, 0,0518 g hydrogen, 0,2247 g nitrogen and 0,2981 g phosphorus. The rest was oxygen. Calculate the number of moles of each element present in the sample of ATP. (Express answers correct to 4 decimal places.) (7)
12.2 The empirical formula of ATP is C10H16O13N5P3. If the relative molecular mass of ATP is 507, what is its molecular formula? (3) [10]
Question 13: Long question
When solutions of potassium chloride (KCl) and silver nitrate (AgNO3) are mixed together, a precipitate of silver chloride is formed.
13.1 Write down a balanced equation for this reaction. (3)
13.2 In a certain experiment, 24 cm3 of 0,05 mol.dm–3 silver nitrate solution exactly reacts with 15 cm3 of potassium chloride solution. How many moles of KCl would react with the given amount of AgNO3? (5)
13.3 Calculate the concentration of the potassium chloride solution. (3) [11]
Question 14: Long questions
You are given three test tubes, labelled A, B and C, each containing an unknown sodium salt. The following observations were made during a practical investigation to identify the salt in each test tube.
- When the salt in C was dissolved in water and silver nitrate (AgNO3) was added, a yellow precipitate formed. This precipitate did not dissolve when an ammonium hydroxide solution was added to it.
- When the salt in A was dissolved in water and barium chloride (BaCl2) was added, a white precipitate formed.
- The salt in B dissolved in HCl and a gas was released.
14.1 Use the above information to identify the salt in each of the test tubes A, B and C. In each case explain how you made your choice. (7)
14.2 Write a balanced chemical equation for the reaction that took place in test tube A when barium chloride was added. (2)
14.3 Write a balanced chemical equation for the reaction that took place in test tube B when HCl was added. (2) [11]
Answers
1.1 D
1.2 C
1.3 C
1.4 C
1.5 D
1.6 B
1.7 A
1.8 A
1.9 B
1.10 B
1.11 B
1.12 B
1.13 D
1.14 A
1.15 D
2.1 True
2.2 False. The mass ratio of carbon to oxygen in a molecule of CO2 will always be 3:8.
2.3 True
2.4 False. The SI unit for measuring quantity of substance is mole. OR The SI unit for measuring mass is kilogram.
2.5 False. The number that is equal to one mole of a substance is Avogadro’s num- ber.
3.1 cation
3.2 barium sulphate
3.3 empirical
3.4 (l)
3.5 chemical
Question 4: Matching pairs
4.1 F
4.2 H
4.3 J
4.4 I
4.5 D
4.6 A
4.7 E
4.8 B
4.9 G
4.10 C
Question 5: Long questions
5.1
- N 30,4% O 69,6%
In 100 g of compound; 30,4 g is N and 69,6 g is O. Therefore, the mole ratio in 100 g is:
N 30,4 = 2,17 O 69,9 = 4,35
14 16
Reduced to whole numbers:
N 2,17 = 1 O 4,35 = 2
2,17 2,17
Ratio N:O = 1:2
Empirical formula is NO2.
5.2
- Formula mass of NO2
= (14 + 16 + 16) = 46
92 = 2
46
Therefore, molecular formula = N2O4.
Question 6: Long questions
6.1 Temperature 0 °C (or 273 K) Pressure 101, 3 kPa = 1,013 × 105 Pa
6.2
- nCO2 = Vol = 33,6 = 1,5 moles
22,4 22,4
According to the equation, 2 mol HCl reacts with 1 mol CaCO3
Therefore, 1,5 mol HCl reacts with x mol Ca CO3
x = 1,5 = 0,75 mol CaCO3
2
Mass of CaCO3 = n × M = 0,75 × (40 + 12 + 48)
Mass CaCO3 = 75 g
Question 7: Long questions
7.1
- nKOH = mass = 10 = 0,179 mol
m (39 + 16 + 1)
According to the equation, 2 mol KOH → 1 mol K2SO4
Therefore, 0,179 mol KOH → ( 0,179 × 1) mol K2SO4
2
= 0,089 mol K2SO4
Mass = n × M = 0,089 × (2 × 39 + 32 + 64) = 15,5 g K2SO4
7.2
- nMg = mass = 0,4 = 0,167 mol
M 24
According to the equation, 1 mol magnesium → 1 mol hydrogen
Therefore, 0,167 mol hydrogen would be produced
Volume at STP = n × 22,4 = 0,167 × 22,4 = 3,74 dm3
Question 8: Long questions
8.1
- M of Al = 27 M of O = 16
M of Al2O3 = (27 + 27 + 16 + 16 + 16) = 102
% Al = 54 × 100 = 52,9%
102
8.2
- n = c × v = 0,1 × 0,25 = 0,025 mol
Mass = n × M = 0,025 × [54 +(32 × 3) + (16 × 12)] = 0,025 × 342
Mass = 8,55 g
Question 9: Long questions
9.1 A precipitate is a solid that forms when two solutions are mixed and form an insoluble salt.
9.2.1 KCl(aq) + NaNO3(aq) → KNO3(aq) + NaCl(aq)
9.2.2 CaSO4(aq) + (NH4)2CO3 → CaCO3(s) + (NH4)2SO4(aq)
9.2.3 BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl(aq)
9.2.4 K2SO4(aq) + Pb(NO3)2(aq) → 2 KNO3(aq) + PbSO4(s)
9.3.1 No precipitate
9.3.2 CaCO3
9.3.3 BaSO4
9.3.4 PbSO4
Question 10: Long questions
10.1 Add some HCl to a small amount of each of the three samples. The one that bubbles and dissolves is the magnesium carbonate. Take the remaining two samples and make them into solutions. Add some AgNO3 solution to each one.A creamy yellow precipitate forms in both. Add some NH4OH solution to each. The test tube containing the precipitate that dissolves when treated with the ammonium solution is the bromide. Therefore, the other tube must be the potassium iodide.
10.2 MgCO3 + 2 HCl → MgCl2 + CO2 + H2O
- correct reactants
- correct products
- balanced correctly
Question 11: Long questions
11.1
- M of Ca = 40 M of O = 16
Mass ratio of Ca:O in CaO must be 40:16 or 5:2
But we have 4,5 g Ca, so mass ratio Ca:O = 4,5:x 5 x = 2 × 4,5 (cross multiply)
x = 2 × 4,5 / 5
= 1,8 g O
Therefore, total mass of CaO formed = 4,5 + 1,8 = 6,3 g CaO
OR
2 Ca + O2 → 2 CaO
2 mol Ca → 2 mol CaO
So 1 mol Ca → 1 Mol CaO
40 g Ca → 56 g CaO
4,5 g Ca → x g CaO
x = 4,5 × 56 = 6,3 g CaO
40
11.2
- CH4 + 2 O2 → CO2 + 2 H2O
2 Al + 3 Cl2 → 2 AlCl3
6 CO2 + 6 H2O → C6H12O6 + 6 O2
Question 12: Long questions
12.1 Mass of oxygen = 1,6270 g – (0,3853 + 0,0518 + 0,2247 + 0,2981) = 0,6671 g
n = mass
M
carbon n = 0,3853 = 0,0321 mol
12
hydrogen n = 0,0518 = 0,0518 mol
1
nitrogen n = 0,2247 = 0,0161 mol
14
phosphorus n = 0,2981 = 0,0096 mol
31
oxygen n = 0,6671 = 0,0417 mol
16
12.2 M of ATP = (10 × 12 + 16 × 1 + 13 × 16 + 5 × 14 + 3 × 31) = 507
Relative formula mass of empirical formula = 507
Relative molecular mass = 507
The molecular formula is the same as the empirical formula.
Question 13: Long questions
13.1 KCl(aq) + AgNO3(aq) → AgCl(s) + KNO3(aq)
13.2
- number of moles of AgNO3 = c × v
= 0,05 × 0,024
= 0,0012 mol
According to the equation, 1 mol of KCl reacts with 1 mol AgNO3.
Therefore, 0,0012 mol KCl is required.
13.3 c = n = 0,0012 = 0,08 mol.dm-3
v 0,015
Question 14: Long questions
14.1
- C – Yellow precipitate formed when AgNO3 is added does not dissolve in NH4OH
Must be an iodide (sodium iodide) - A – A white precipitate formed when barium chloride is added indicates a sulphate.
Must be sodium sulphate. - B – A substance that dissolves in acid and forms a gas indicates the production of CO2
Must be sodium carbonate.
14.2 BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2 NaCl(aq)
14.3 Na2CO3(aq) + 2 HCl(aq) → 2 NaCl(aq) + CO2(g) + H2O(l)