PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2018
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2018
MEMORANDUM
QUESTION 1
1.1 C ✔✔ (2)
1.2 D ✔✔ (2)
1.3 B ✔✔ (2)
1.4 C ✔✔ (2)
1.5 B ✔✔ (2)
1.6 B ✔✔ (2)
1.7 B ✔✔ (2)
1.8 C ✔✔ (2)
1.9 A ✔✔ (2)
1.10 D ✔✔ (2) [20]
QUESTION 2
2.1
2.1.1 A ✔ (1)
2.1.2 B ✔ (1)
2.1.3 D ✔ (1)
2.1.4 D✔ (1) [4]
2.2
2.2.1 Butanal✔ (1)
2.2.2
- 5-ethyl-6,6-dimethyloctan-3-ol
OR/OF - 5-ethyl-6,6-dimethyl-3-octanol (4)
Marking criteria:
- Stem, i.e. octan.✔
- Correct functional group, i.e. -ol.✔✔
- Two methyl groups and one ethyl group.✔
- Correct numbering of substituents and functional group ✔
IF:
- Any error e.g. hyphens omitted and/or incorrect sequence: Max..3/4
2.3 Compounds with the same molecular formula, ✔ but different positions of the side chain/substituents/functional groups on parent chain. ✔ (2)
2.4
2.4.1 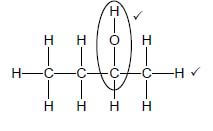 (2)
(2)
Marking criteria:
- Whole structure correct: 2/2
- Only functional group correct: Max.: 1/2
IF:
- More than one functional group: 0/2
2.4.2 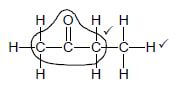 (2)
(2)
Marking criteria:
- Whole structure correct: 2/2
- Only functional group correct : Max.: 1/2
IF:
- More than one functional group:
2.4.3 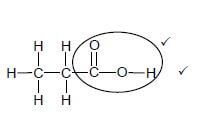 (2)
(2)
Marking criteria:
- Whole structure correct: 2/2
- Only functional group correct : Max.: 1/2
IF:
- More than one functional group: [17]
QUESTION 3
3.1 150 kPa✔ (1)
3.2
3.2.1 The temperature at which the vapour pressure equals atmospheric/external pressure. ✔✔( 2 or 0) (2)
3.2.2 55 °C ✔ (1)
3.3
3.3.1 Z ✔ (1)
3.3.2
- Carboxylic acids have, in addition to London forces and dipole-dipole forces, two sites for hydrogen bonding between molecules. ✔
OR
Carboxylic acids can form dimers due to strong hydrogen bonding between molecules. - Alcohols have, in addition to London forces and dipole-dipole forces, one site for hydrogen bonding between molecules. ✔
- Ketones has, in addition to London forces, dipole-dipole forces between molecules. ✔
- Intermolecular forces in carboxylic acids is the strongest./Most energy needed to overcome/break intermolecular forces in ethanoic acid. ✔ (4)
3.3.3
- Propanone✔
OR - Propan-2-one
OR - 2-propanone (1) [10]
QUESTION 4
4.1 The chemical process in which longer chain hydrocarbon molecules are broken down to shorter more useful molecules. ✔ (2)
4.2
4.2.1 III ✔ (1)
4.2.2 II ✔ (1)
4.2.3 I ✔ (1)
4.3
4.3.1 Heat/Light /UV light ✔ (1)
4.3.2 P or S ✔ (1)
4.3.3 Ethene ✔ (1)
4.3.4 C8H18 ✔✔ (Correct Structural formula : 1/2) (2)
4.3.5 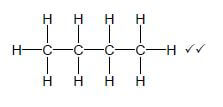 (2)
(2)
Marking criteria:
- Whole structure correct: 2/2
- 4 C atoms in chain: Max : 1/2
- Correct condensed formula 1/2
4.3.6 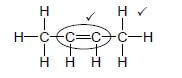 (2)
(2)
Marking criteria:
- Whole structure of alkene/haloalkane correct: 2/2
- Only functional group correct: 1/2
- Correct condensed structure CH3CH=CHCH3 1/2 [14]
QUESTION 5
5.1 ONLY ANY ONE OF:
- Change in concentration ✔ of a reactant/product per unit time.
- Rate of change in concentration. ✔✔
- Change in amount/number of moles/volume/mass of products/reactants per (unit) time ✔
- Amount/number of moles/volume/mass of products formed OR reactants used per (unit) time. ✔ (2)
5.2 More than ✔
- Accept/
Equal to (1)
5.3 Graph of average reaction rate versus volume of Na2S2O3(aq)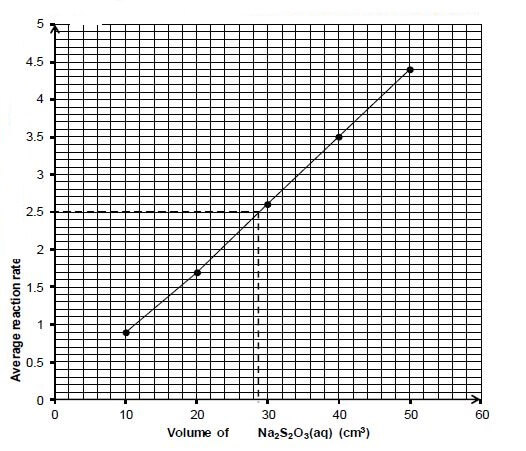 (3)
(3)
Marking criteria | |
Any 3 points correctly plotted. | ✔ |
All (5) points correctly plotted. | ✔ |
Straight line drawn. | ✔ |
5.4
5.4.1 Marking criteria:
- y axis: 2,5 x 10-2 s-1 ✔
- Dotted line drawn from the y-axis to the x-axis as shown. ✔
- V = 28 to 30 cm3 ✔ (3)
5.4.2 Criteria for conclusion:
- Dependent and independent variables correctly identified. ✔
- Relationship between the independent and dependent variables correctly stated ✔
Examples:
- Reaction rate of reaction increases with an increase in concentration/volume of sodium thiosulphate.
- Reaction rate decreases with a decrease in concentration/volume of sodium thiosulphate.
- Reaction rate is (directly) proportional to concentration/volume of sodium thiosulphate.
5.5
- More( Na2S2O3) particles per unit volume. ✔
- More effective collisions per unit time./Higher frequency of effective collisions. ✔
- Increase in reaction rate.✔
5.6
| OPTION 1 n(S)produced/gevorm = m M = 1,62 32 = 0,0506 mol n(Na2S2O3) = n(S) = 0,0506 mol | Marking criteria:
|
OPTION 2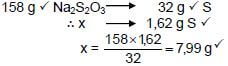 [Range: 7,90 to 8,06] | |
(4) [18]
QUESTION 6
6.1
6.1.1 When the equilibrium in a closed system is disturbed, the system will re-instate a new equilibrium by favouring the reaction that will oppose the disturbance. ✔✔ (2)
6.1.2
- Percentage yield increases with an increase in temperature. ✔
- Forward reaction is favoured.
- Increase in temperature favours an endothermic reaction. (3)
6.1.3 When the pressure increases, the reaction that leads to a decrease in the number of moles will be favoured. ✔✔
Accept
- When the pressure increases, the yield increases ✔ because the equilibrium position shifts to the right. ✔ (2)
6.1.4 I ✔✔ (2)
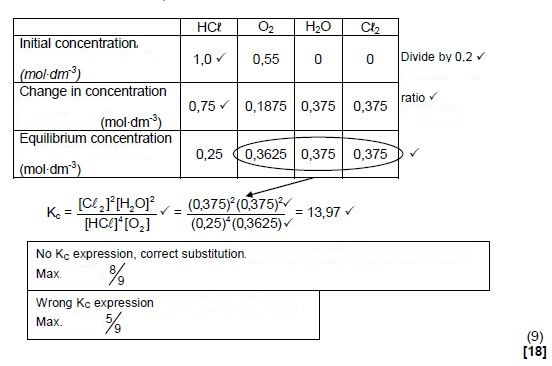
6.2 Mark allocation
- Substitution of 36,5 g∙mol-1 in n = m/M . ✔
- Change n(HCℓ) = initial - equilibrium ✔
- USING ratio: 4 : 1 : 2 : 2 ✔
- Equilibrium: n(O2) & n(H2O) & n(Cℓ2) = initial ± change ✔
- Divide by volume (0,2 dm3) ✔
- Correct Kc expression (formulae in square brackets). ✔
- Substitution of reactant concentrations ✔
- Substitution of product concentrations.✔
- Final answer: 13,966 to/tot 18,72 ✔
Range: 13,966 to/tot 18,72
OPTION 1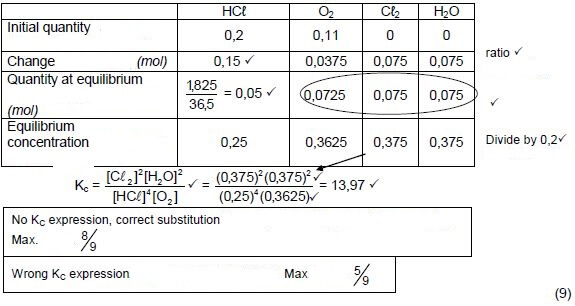 (9)
(9)
OPTION 2:
- n(HCℓ)equilibrium = m/ M = 1,825/36,5 = 0,05 mol
n(HCℓ)reacted= 0,2 - 0,05 = 0,15 mol ✔- n(O2)reacted = ¼n(HCℓ)reacted= ¼ x 0,15 = 0,0375 mol
n(Cℓ2)formed = ½n(HCℓ)reacted = ½ x 0,15 = 0,075 mol
n(H2O)formed = ½n(HCℓ)reacted/= ½ x 0,15 = 0,075 mol
Using ratio ✔
- n(O2)reacted = ¼n(HCℓ)reacted= ¼ x 0,15 = 0,0375 mol
- n(O2)equilibrium = 0,11 - 0,0375 = 0,0725 mol
n(Cℓ2)equilibrium = n(H2O)equilibrium = 0,075 mol
c(O2)equilibrium = n/V = 0,0375/0,2 = = 0,3625 mol∙dm-3
c(Cℓ2)equilibrium/= c(H2O)equilibrium = n/V
= 0,075/0,2 = 0,375 mol∙dm-3 - Kc = [H2O]2[CL2]2 = (0,375)2(0,375)2 = 13,97
[HCL]4[O2] (0,25)4(0,3625)
No KC expression, correct substitution: Max. 8/9
Wrong KC expression :Max. 5/9 (9)
CALCULATIONS USING CONCENTRATIONS
Mark allocation
- Substitution of/Vervanging van 36,5 g∙mol-1 n = m/ M
- Initial concentration of reactants: c(HCℓ) = 1,0 & c(O2) = 0,55 mol∙dm-3
- Change: c(HCℓ) = 0,75 mol∙dm-3 (initial - equilibrium)
- USING ratio : 4 : 1 : 2 : 2
- Equilibrium : c(H2O) = c(Cℓ2) = 0,3625 mol∙dm-3 (initial+change) and c(O2) = 0,3625 mol∙dm-3 (initial - change)
- Correct Kc expression (formulae in square brackets).
- Substitution of reactant concentrations
- Substitution of product concentrations.
- Final answer: 13,97
Range: 13,966 to 18,72
OPTION 3
- n(HCℓ)equilibrium = m/ M
= 1,825/36,5
= 0,05 mol
QUESTION 7
7.1
7.1.1
- H2O ✔
- HSO4 ✔ (2)
7.1.2
- Strong ✔
- Completely ionised (in water)✔ (2)
7.2
7.2.1 Marking Criteria:
- Formula/Formule: ca × Va = na /c = n
ca × Vb nb V - Substitute 0,15 x 24 OR/OF 0,15 x 0,024 ✔
- Use 26 cm3 OR 0,026 dm3 ✔
- Use mole ratio : 1:2 ✔
- Final answer : 0,28 mol∙dm-3 ✔ (0.2769… mol∙dm-3)
OPTION 1
ca × Va = na
ca × Vb nb
0,15 × 24 = 1
cb × 26 2
c(NaOH) = 0,28 mol.dm3
OPTION 2
n(H2SO4) = cV
= (0,15)(0,024)
= 3,6 x 10-3 mol
n(NaOH) =2(3,6 x 10-3)
= 7,2 x 10-3 mol
c = n
V
= 7,2 x 10-3
0,026
= 0,28 mol∙dm-3 (5)
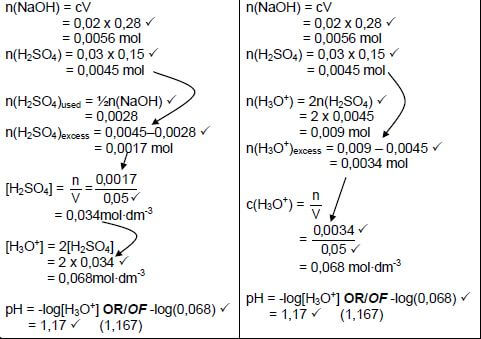
7.2.2 Marking Criteria
- Calculate n(NaOH): 0,02 x 0,28✔
- Calculate n(H2SO4): 0,03 x 0,15 ✔
- Use ratios: n(H2SO4) = ½n(NaOH) ✔
- n(H2SO4)excess = n(H2SO4)initial - n(H2SO4)used = 0,0045 - 0,0028 ✔
- Substitute 0,05 dm3 in c = n
V ✔ - Substitution 2 x 0,034 in 2[H2SO4] ✔
- Formula: -log[H3O+] OR Substitute: -log(0,068) ✔
- Final answer: 1,10 to/tot 1,167 ✔
| Option 1 | Option 2 |
 | |
(8)[17]
QUESTION 8
8.1
8.1.1 A substance that loses/donates electrons ( 2 or 0) ✔✔ (2)
8.1.2 Platinum/Pt ✔ (1)
8.1.3 Sn2+(aq)/tin(II) ions ✔ (1)
8.1.4
- Pt | Sn2+(aq) , Sn 4+(aq) || Ag+(aq) | Ag(s)
OR - Pt| Sn2+(1 mol∙dm-3) ,Sn 4+ (1 mol∙dm-3) || Ag+ (1 mol∙dm-3) | Ag(s)
ACCEPT - Pt| Sn2+ | Sn 4+ || Ag+ | Ag (3)
8.1.5
OPTION 1 E°cell = E°reduction - E°oxidation | Notes
|
| Option 2 Ag+(aq) + e- → Ag(s) 0,80V Sn2+(aq) → Sn4+(aq) + 2e- - 0,15V 2Ag+(aq) + Sn2+(aq) → Sn4+(aq) + 2Ag(s) 0,65V | |
(4)
8.2
8.2.1 Magnesium becomes smaller./Brown solid forms/Mg disappears/eaten away/Mg changes colour. ✔ (1)
8.2 2
- Cu2+ is a stronger oxidising agent ✔(than Mg2+) and will be reduced to ✔ Cu. ✔
OR - Mg is a stronger reducing agent (than Cu) and will reduce Cu2+ to Cu. (3) [15]
QUESTION 9
9.1
- The chemical process in which electrical energy is converted to chemical energy. ✔✔
OR - The use of electrical energy to produce a chemical change. (2)
9.2 B ✔ (1)
9.3
- Cu2+(aq) + 2e- → Cu ✔✔ (2)
Marking criteria
- Cu ← Cu2+(aq) + 2e- ( 2/2 ) Cu2+(aq) + 2e- ⇌ Cu ( 1 /2 )
Cu ⇌ Cu2+(aq) + 2e-( 0/2 ) Cu2+(aq) + 2e- ← Cu ( 0/2 ) - Ignore if charge omitted on electron.
- If charge (+) omitted on Cu2+
Max: 1/2
9.4
- % purity = m(Cu) × 100
m(Cu )impure
= 4,4 ×100
5
= 88% ✔ (4)
Marking criteria:
- Substitute 4,4 ✔
- Substitute 5 ✔
- x 100 ✔
- Final answer: 88% ✔ [9]
QUESTION 10
10.1
10.1.1 N2(g) + 3H2(g) ✔ → 2NH3(g) ✔ bal ✔
Notes:
- Reactants ✔ Products ✔ Balancing ✔
- Ignore if phases are omitted
- Ignore ⇌
- Marking rule/Nasienreël 3.9 (3)
10.1.2 (NH4)2SO4 ✔ (1)
10.1.3 Ostwald process ✔ (1)
10.1.4 Ammonium nitrate ✔ (1)
10.2
10.2.1
- The ratio of nitrogen (N), phosphorous (P) and potassium (K) in a certain fertiliser.✔
Accept :
- nitrogen, phosphorous and potassium ✔ (1)
10.2.2 Percentage fertiliser in the bag.✔ (1)
10.2.3 OPTION 1
- % K =5 ✔ x 22% ✔
12
= 9,17%
∴ m(N) = 9,17 × 10 kg
100
= 0,92 kg ✔
OPTION 2
- m(nutrients):
22/100 × 10 = 2,2 kg
∴ m(K) = 5/12 (2,2)
= 0,92 kg ✔ (4)
[12]
TOTAL: 150