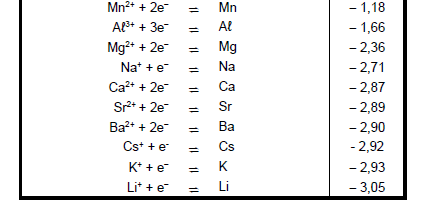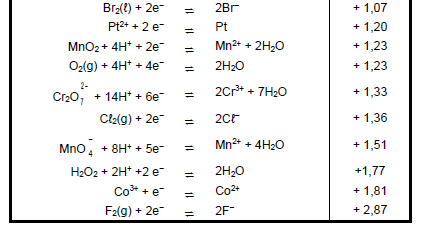PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - PAST PAPERS 2017 JUNE
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE
JUNE 2017
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of EIGHT questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number your answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four possible options are provided as answers to the following questions. Each question has only ONE correct answer. Choose the answer and write only the correct letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for e.g. 1.11 E.
1.1 A potential energy diagram for a hypothetical reaction is given below:
X2(g) → 2 X(g)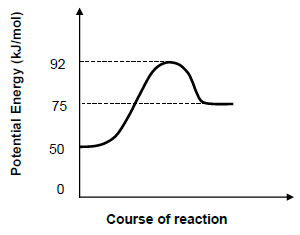
Course of reaction
The ΔH for the reaction in kJ/mol is …
- 17.
- -17.
- 25.
- -25. (2)
1.2 Which pair of compounds represents UNSATURATED hydrocarbons?
- Alkenes and Alkynes
- Alkanes and Alkynes
- Alkanes and Alkenes
- Alcohols and Alkenes (2)
1.3 An atom, group of atoms or a bond that gives a group of organic compounds its characteristic physical and chemical properties is called a …
- polymer.
- monomer.
- functional group.
- homologous series. (2)
1.4 Which ONE of the following statements is always TRUE about the relationship between strength of intermolecular forces and boiling point?
- Boiling point is directly proportional to the strength of intermolecular forces.
- As the strength of intermolecular forces increases boiling point increases.
- As the strength of intermolecular forces increases boiling point decreases.
- As the strength of intermolecular forces increases the boiling point is not affected. (2)
1.5 In the flow diagram below, P and Q represent two organic compounds.
Which ONE of the following is the CORRECT condensed molecular formula for compound Q?
- CH2CH2
- CH3CH3
- CH3CH2Br
- CH3CH2OH (2)
1.6 In a homogeneous reaction the reactants and products are always …
- gases.
- liquids.
- solids.
- in the same phase. (2)
1.7 Which ONE of the following changes will NOT INFLUENCE the rate at which oxygen is produced?
2 H2O2 (aq) →2 H2O(ℓ) + O2(g)
- Increase pressure
- Increase temperature
- Add a suitable catalyst
- Increase the concentration of H2O2 (2)
1.8 The following graph shows the relationship between the equilibrium constant Kc and the quantity X for the hypothetical reaction:
A2(g) + B2(g) ⇌ 2AB(g)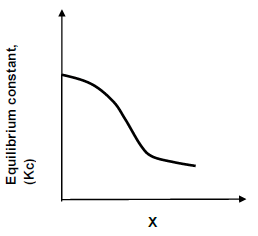
What quantity is represented by X on the horizontal axis?
- Mass
- Pressure
- Temperature
- Concentration (2)
1.9 A strong acid is titrated with a base. The dissociation constant for the base, Kb is 2,8 x 10-6 at 25 ºC.
Which ONE of the following indicators is most suitable for the titration?
| Indicator | pH range over which indicator changes colour |
| A | 4,2 to 6,2 |
| B | 6,0 to 7,6 |
| C | 8,0 to 9,6 |
| D | 10,0 to 12 (2) |
1.10 Chromate (yellow solution) and dichromate ions (orange solution) are in equilibrium with each other in an aqueous solution according to the following balanced equation:
2CrO42-(aq) + 2H+(aq) ⇌ Cr2O72- (aq) + H2O(ℓ)
Yellow Orange
What ONE of the following changes should be made to change the colour of the solution to orange?
- Add more H2O
- Lower the pH
- Increase the pH
- Increase [Cr2O72-] (2)
[20]
QUESTION 2
The letters A to H in the table represent eight organic compounds:
A. 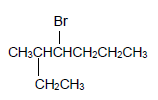 | B. O |
| C. C4H10O | D. Methyl ethanoate |
| E. 2,3-dimethylbutane | F. 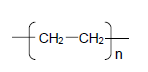 |
| G. Ethanoic acid | H. CnH2n |
2.1 Write down the letter that represents a compound that is:
2.1.1 A proton donor (1)
2.1.2 A large molecule composed of small monomer units covalently bonded in a repeating pattern (1)
2.2 Write down the:
2.2.1 GENERAL FORMULA of the homologous series to which compound E belongs (1)
2.2.2 NAME of the functional group found in compound G (1)
2.2.3 EMPIRICAL FORMULA of compound H (1)
2.3 Write down the IUPAC name of:
2.3.1 Compound A (3)
2.3.2 Compound B (2)
2.3.3 A FUNCTIONAL ISOMER of compound G (2)
2.4 Write down the STRUCTURAL FORMULA of compound E. (2)
2.5 Compound D is prepared from the reaction of a carboxylic acid and an alcohol in the presence of an inorganic acid catalyst. A water bath is used to heat the reaction mixture.
Write down the:
2.5.1 NAME or FORMULA of the inorganic acid catalyst (1)
2.5.2 STRUCTURAL FORMULA of compound D (2)
2.5.3 Property of alcohols that make it necessary to use a water bath to heat the reaction mixture instead of direct heat (1)
2.6 Compound C is a TERTIARY alcohol.
Write down the STRUCTURAL FORMULA and IUPAC name of compound C. (4)
2.7 Write down the MOLECULAR FORMULAE of the TWO products formed during the complete combustion of compound E. (2)
[24]
QUESTION 3
Learners use alcohols A to C to investigate a factor that influences boiling points of alcohols.
3.1 Define the term boiling point. (2)
| Compounds | Alcohols |
| A | CH3OH |
| B | CH3CH2OH |
| C | CH3CH2CH2OH |
3.2 For this investigation write down the:
3.2.1 Independent variable (1)
3.2.2 Apparatus used to measure the boiling point (1)
3.3 Which ONE of the three compounds will have the HIGHEST boiling point? (1)
3.4 Explain your answer to QUESTION 3.3. (3)
3.5 The learners now compare the boiling points of compounds D and E, shown in the table below. Compounds D and E belong to different homologous series.
| Compounds | Boiling point(°C) | |
| D | Ethanol | 78,1 |
| E | Ethanal | 20,2 |
3.5.1 Define the term homologous series. (2)
3.5.2 Explain fully why the boiling point of compound D is HIGHER than that of compound E. (4)
3.5.3 Which ONE of the compounds D or E will have a HIGHER vapour pressure? Use information from the table to give a reason. (2)
[16]
QUESTION 4
The flow diagram below shows three organic reactions that involve the compound
2-bromobutane.
Reaction A: Alkane + Y → 2-bromobutane + HBr
Reaction B: 2-bromobutane + KOH → Compound X + KBr + H2O
Reaction C: 2-bromobutane + KOH → Alcohol + KBr
4.1 Write down the type of reaction represented by:
4.1.1 Reaction A (1)
4.1.2 Reaction B (1)
4.2 For reaction A, write down the:
4.2.1 NAME or FORMULA of the inorganic reagent Y (1)
4.2.2 One reaction condition needed for the reaction to take place (1)
4.2.3 IUPAC name of the alkane (2)
4.3 Write down the STRUCTURAL FORMULA of compound X the major organic product produced in reaction B. (2)
4.4 Write down the STRUCTURAL formula of the alcohol produced in reaction C. (2)
4.5 In both reactions B and C the same inorganic reagent KOH is used. Write down TWO reaction conditions that will favour reaction C over reaction B. (2)
[12]
QUESTION 5
A group of learners uses the reaction of hydrochloric acid with magnesium ribbon to investigate the factors that influence rate of reaction. The balanced equation for the reaction is given below:
Mg(s) + 2 HCℓ (aq) → MgCℓ2(aq) + H2(g)
The hydrochloric acid is in EXCESS and the same mass of magnesium is used in ALL the experiments.
| Experiment | REACTION CONDITIONS | |||
| Concentration of HCℓ (aq) (mol.dm-3) | Temperature (ºC) | State of division of 0,24 g Magnesium | ||
| Before | After | |||
| 1 | 2 | 35 | 57 | powder |
| 2 | 2 | 30 | 48 | ribbon |
| 3 | 2 | 20 | 33 | ribbon |
| 4 | 1,5 | 30 | 45 | ribbon |
5.1 Define reaction rate. (2)
5.2 In which experiment is the reaction rate HIGHEST? Give TWO reasons. (3)
5.3 The reaction in Experiment 2 is compared to the reaction in Experiment 4.
5.3.1 Write down ONE control variable for this comparison. (1)
5.3.2 How does the amount of hydrogen gas produced in Experiment 2 compare to the amount produced in Experiment 4 if the same volume of acid is used in both experiments?
Write down only HIGHER THAN, SMALLER THAN or EQUAL TO.
Give a reason for your answer. (2)
5.4 Give a reason why it is not a fair test to compare the rate of reaction of
Experiment 1 with that of Experiment 3. (1)
5.5 Calculate the mass of hydrochloric acid that remains in the flask at the completion of the reaction in Experiment 1 if the initial volume of the hydrochloric acid is 80 cm3. (7)
5.6 The Maxwell-Boltzman distribution curves labelled P, Q and R for the reactions in experiments 1, 2 and 3 in random order are shown below.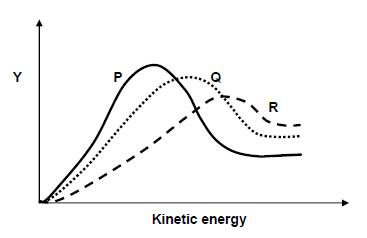
5.6.1 Write down the name of the label, Y, on the vertical axis. (1)
5.6.2 Which curve (Q, P or R) represents the results of Experiment 3? (1)
5.6.3 With the aid of the collision theory explain the effect of temperature on reaction rate. (4)
[22]
QUESTION 6
The following reaction reaches chemical equilibrium in a sealed container at 70 ºC.
N2O4 (g) ⇌ 2 NO2 (g)
6.1 Define the term chemical equilibrium. (2)
6.2 What effect will the following changes have on the number of moles of NO2 at equilibrium?
Write down only INCREASES, DECREASES or REMAINS UNCHANGED.
6.2.1 Adding more N2O4 into the container. (1)
6.2.2 Increasing the pressure by decreasing the volume. (1)
6.3 Explain the answer to QUESTION 6.2.2 above by referring to Le Chatelier’s principle. (3)
6.4 The following graph shows the changes in the concentration of N2O4 against time.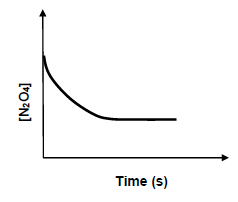
Redraw the graph on the same set of axes use a dotted line to sketch a graph that would be obtained when a catalyst is added to the reaction mixture at the start of the reaction. (2)
6.5 The table below gives the equilibrium constant values Kc for the reaction at different temperatures.
| Temperature(°C) | Kc |
| 23 | 8,03 |
| 70 | 0,32 |
| 100 | 0,067 |
6.5.1 At which temperature is the yield of NO2 highest? Give a reason. (2)
6.5.2 When the reaction establishes equilibrium at 70 ºC it is found that the concentration of N2O4 in the equilibrium mixture is 0,5 mol.dm-3.
Calculate the initial concentration of N2O4. (7)
6.5.3 Is the forward reaction ENDOTHERMIC or EXOTHERMIC?
With the aid of information from the table and Le Chateliers’ principle, fully explain the answer. (4)
[22]
QUESTION 7
7.1 Ethanoic acid is a monoprotic acid that ionises in water according to the equation.
CH3COOH(ℓ) + H2O(ℓ) ⇌ CH3COO- (aq) + H3O+(aq) Ka = 1,8 x 10-5 at 25 ºC
7.1.1 Define the term monoprotic acid. (2)
7.1.2 Write down the NAME or FORMULA of the conjugate base of ethanoic acid. (1)
7.1.3 Is ethanoic acid a STRONG or WEAK acid?
Refer to the given information to give a reason. (2)
7.2 A sodium hydroxide solution (NaOH) has a concentration of 1 x 10-5 mol.dm-3.
Calculate the:
7.2.1 pH of the solution (4)
7.2.2 Volume to which 10 cm3 of the sodium hydroxide solution must be diluted to obtain a solution with a concentration of 1 x 10-6 mol.dm-3 (3)
7.3 A certain compound has sodium carbonate (Na2CO3) as the main ingredient.
To determine the amount of sodium carbonate present in a sample of the compound 100 cm3 of a 0,8 mol.dm-3 solution of sulphuric acid was added to the sample in a flask.
The sulphuric acid solution is in EXCESS.
The equation below shows the reaction taking place in the flask.
Na2CO3(s) + H2SO4(aq) → Na2SO4(aq) + CO2(g) + H2O(ℓ) Reaction 1
7.3.1 Calculate the amount in moles of sulphuric acid added to the flask. (3)
In a titration exactly 35 cm3 of a 0,3 mol.dm-3 potassium hydroxide solution neutralises the excess amount of sulphuric acid left over in Reaction 1 according to the balanced equation shown below.
2 KOH(aq) + H2SO4(aq) → K2SO4(aq) + 2 H2O(ℓ) Reaction 2
7.3.2 Calculate the mass of sodium carbonate present in the sample. (8)
7.4 Write down a balanced equation for the hydrolysis of sodium carbonate (Na2CO3). (3)
[26]
QUESTION 8
The graph of percentage yield of NH3, produced in the reaction given below,
versus pressure at different temperatures is shown below.
N2(g) + 3 H2(g) ⇌ 2 NH3(g)
GRAPH OF PERCENTAGE YIELD AT DIFFERENT TEMPERATURES VERSUS PRESSURE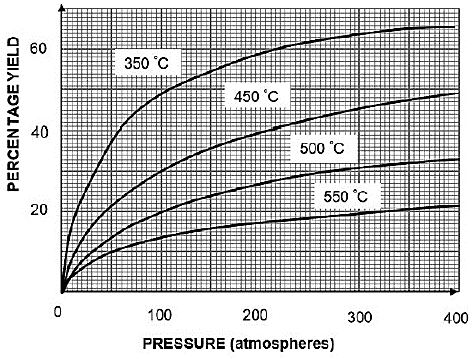
8.1 Write down:
8.1.1 In words the relationship between temperature and percentage yield at constant pressure for this reaction (2)
8.1.2 The percentage yield at a temperature of 350 ºC and a pressure of 100 atmospheres (1)
8.1.3 The pressure at which the percentage yield is 40% at 450 ºC (1)
8.2 Exactly 112 grams of nitrogen gas was allowed to react with hydrogen gas in a closed container. The reaction reached equilibrium at a temperature of 350 ºC and a pressure of 200 atmospheres.
Calculate the actual yield (in moles) of NH3. (4)
[8]
TOTAL: 100
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
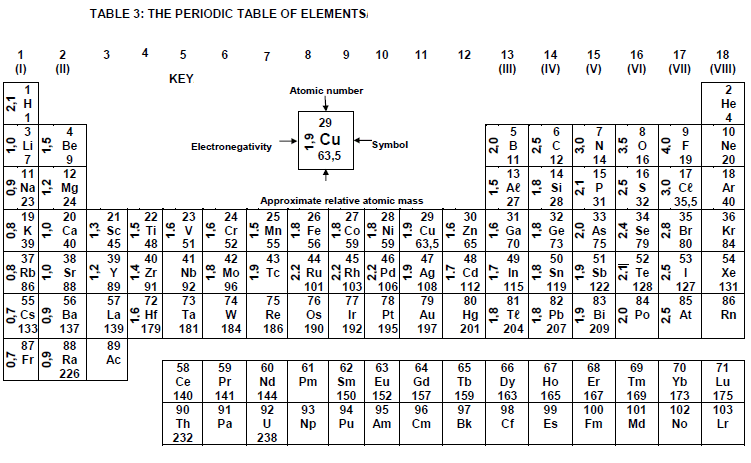
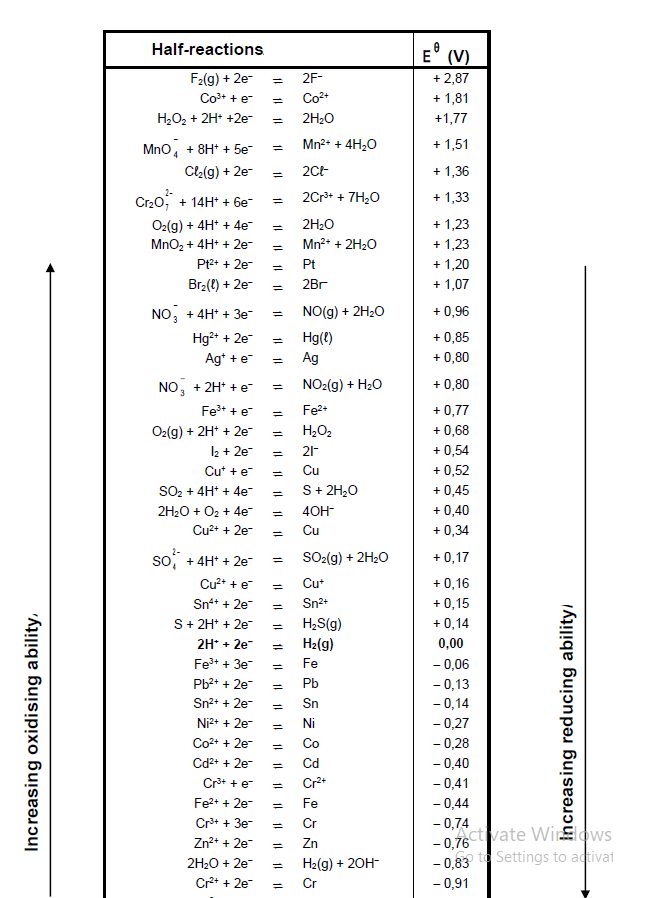
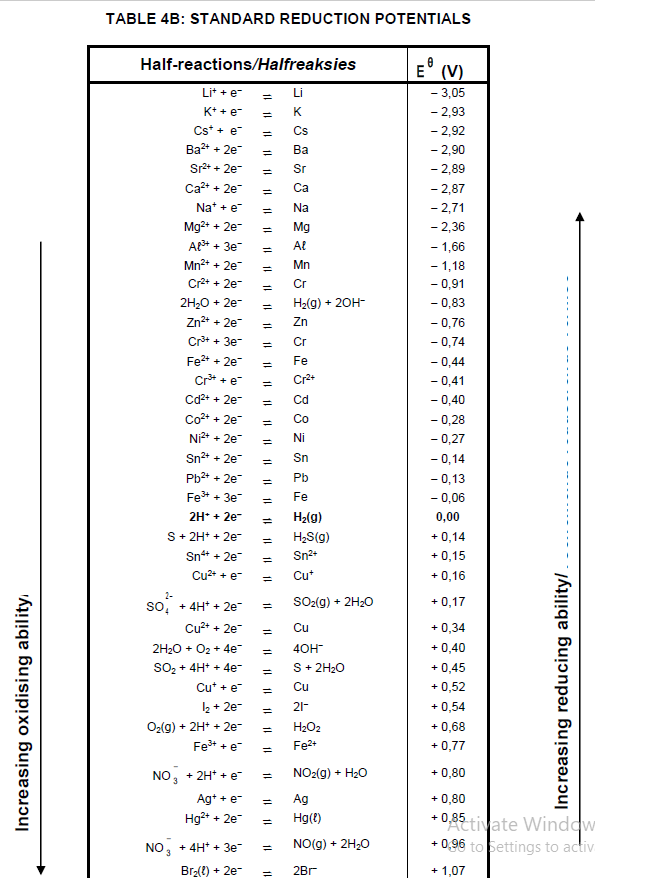
TABLE 4A: STANDARD REDUCTION POTENTIALS