PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - AMENDED SENIOR CERTIFICATE PAST PAPERS AND MEMOS MAY/JUNE 2017
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY PAPER 2
GRADE 12
MEMORANDUM
SENIOR CERTIFICATE EXAMINATIONS
MAY/JUNE 2017
QUESTION 1
1.1 D ✓✓ (2)
1.2 D ✓✓ (2)
1.3 B ✓✓ (2)
1.4 A ✓✓ (2)
1.5 C ✓✓ (2)
1.6 A ✓✓ (2)
1.7 B ✓✓ (2)
1.8 D ✓✓ (2)
1.9 C ✓✓ (2)
1.10 C ✓✓ (2)
[20]
QUESTION 2
2.1 A bond / an atom / a group of atoms ✓ that determine(s) the (physical and chemical) properties of a group of organic compounds. ✓ (2)
2.2
2.2.1 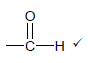 ✓ (1)
✓ (1)
2.2.2 Carboxyl (group)✓ (1)
2.3
2.3.1 Ketones ✓ (1)
2.3.2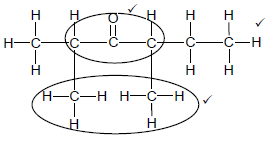
Marking criteria:
- Functional group ✓
- Two methyl substituents ✓
- Whole structure correct: 3
3 (3)
2.4
2.4.1 5-bromo-4-ethyl-2,2-dimethylhexane
Marking criteria:
- Correct stem i.e. hexane.✓
- All substituents (bromo, ethyl and dimethyl) correctly identified.✓
- IUPAC name completely correct including numbering, sequence, hyphens and commas. ✓(3)
2.4.2 4-methylpent-2-yne ✓✓
OR
4-methyl-2-pentyne (2)
| NOTE 4-methyl ✓ pent-2-yne ✓ IUPAC name correct but hyphens omitted: ½ |
[13]
QUESTION 3
3.1
3.1.1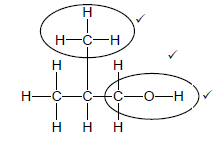
Marking criteria:
Accept:
|
(3)
3.1.2 D ✓
Accept:
butan-1-ol (1)
3.1.3 G ✓
Accept
2-methylpropan-2-ol(1)
3.2
3.2.1 (Increase in) chain length / molecular size / molecular mass/ number of C-atoms/ surface area / contact area / number of electrons✓(1)
3.2.2 London forces / dispersion forces / induced dipole forces ✓(1)
3.3
3.3.1 108 (°C) ✓ (1)
3.3.2 Compare compound F with compounds C and D:
- Compound F has a larger molecular mass / molecular size / surface area/contact area / number of C-atoms / number of electrons / than compound C. ✓
- Compound F is more branched than compound D. ✓
- Intermolecular forces in compound F are stronger than in compound C and weaker than in compound D. ✓
- More energy needed to overcome intermolecular forces in compound F than in compound C and less energy needed to overcome (break) intermolecular forces in compound F than in compound D. ✓(4)
3.4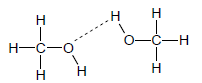
Marking criteria:
|
(2)
3.5
3.5.1 Esterification / Condensation ✓(1)
3.5.2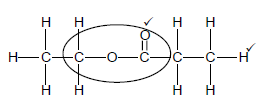
Marking criteria:
|
[17]
QUESTION 4
4.1
4.1.1 Addition / hydrogenation ✓(1)
4.1.2 Substitution / halogenation / chlorination ✓(1)
4.1.3 Elimination / dehydration ✓(1)
4.2 2-bromopropane ✓
Note:
IF:
Bromopropane ½
2-bromo✓
propane✓ (2)
4.3
4.3.1 Dehydrohalogenation / Dehydrobromination ✓(1)
4.3.2 Hot✓ ethanolic strong base✓
- Concentrated strong base / NaOH / KOH✓
OR
Strong base with no water.
OR
Strong base in (pure) ethanol as solvent. - Strongly heated or hot base
OR
High temperature/heat strongly(2)
4.4
4.4.1 H2O / NaOH / KOH ✓ (1)
4.4.2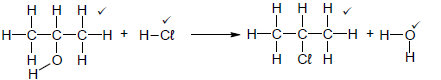
Notes
|
(4)
[13]
QUESTION 5
5.1
5.1.1 To measure volume / amount ✓ of gas/oxygen produced.(1)
5.1.2 Catalyst / Speeds up the reaction. / Increases reaction rate. ✓ (1)
5.2 No gas / bubbles produced.✓
OR
Volume of gas in syringe remains constant. / The plunger stops moving.(1)
5.3 CuO / Copper(II) oxide / catalyst ✓ (1)
5.4
- A catalyst provides an alternative pathway of lower activation energy. ✓
- More molecules have sufficient / enough kinetic energy. ✓
OR - More molecules have kinetic energy equal to or greater than the activation energy.
More effective collisions per unit time. / Frequency of effective collisions increases. ✓(3)
5.5
5.5.1 Released ✓
Products at lower (potential) energy than reactant. / Reaction is exothermic / ΔH < 0 ✓ (2)
5.5.2 B ✓ (1)
5.6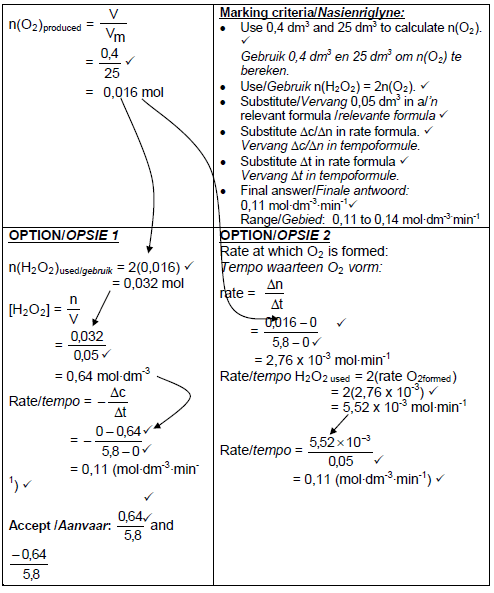
(6)
[16]
QUESTION 6
6.1
6.1.1 Products can be converted back to reactants. ✓
OR
Both forward and reverse reactions can take place.(1)
6.1.2 Endothermic ✓ (1)
6.1.3
- Kc increases with increase in temperature. ✓
- Forward reaction is favoured. / Concentration of products increases. / Concentration of reactants decreases. ✓
- Increase in temperature favours an endothermic reaction. ✓(3)
6.1.4 Increases ✓ (1)
6.1.5 Remains the same ✓ (1)
6.2
6.2.1
Marking criteria:
|
OPTION 1
Use mole ratio No KC expression: Max. 7 Kc = 1 ✓(f) | |||||||||||||||
OPTION 2 No KC expression: Max. 7 |
6.2.2 Remains the same✓ (1)
[16]
QUESTION 7
7.1
7.1.1 Weak (acid)✓ (1)
7.1.2 pH = -log[H3O+] ✓
4 ✓ = -log[H3O+]
[H3O+] = 1 x 10-4 mol∙dm-3 ✓
(3)
7.2
7.2.1 A substance that produces hydroxide ions / OH- in water. ✓✓
NOTE:
If water is omitted: ½ (2)
7.2.2
Marking guidelines:
|
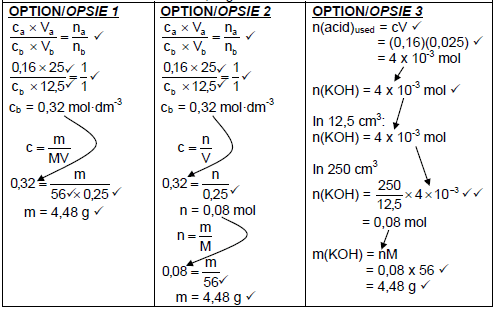 |
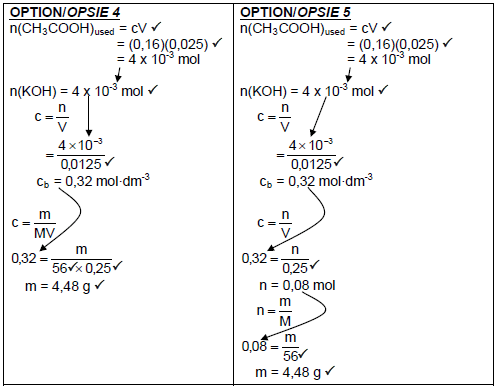 |
(7)
7.2.3 Greater than 7 ✓ (1)
7.2.4 ![]()
Due to formation of (OH-), the solution is basic / alkaline✓
Notes
|
(3)
[17]
QUESTION 8
8.1
8.1.1 Emf ✓(1)
8.1.2 Voltmeter ✓ (1)
8.1.3 Salt bridge ✓ (1)
8.1.4 Temperature : 25 °C / 298 K ✓
Concentration : 1 mol·dm-3 ✓ (2)
8.2
| Marking criteria | |
| Dependent and independent variables correctly identified. | ✓ |
| Relationship between the independent and dependent variables correctly stated | ✓ |
Examples
Emf increases as concentration (of oxidising agent) increases.
NOTE:
IF:Emf is directly proportional to concentration.½ (2)
8.3
| OPTION 1 Eθcell = Eθreduction - Eθoxidation 1,11 ✓ = Eθ+x/x2+ - (- 0,76) ✓ Eθ+x/x2+ = 0,35 (V) ✓ X = Copper ✓ Accept: Cu/Cu2+ half reaction | Notes
|
| OPTION 2 X2+(aq) + 2e- → X(s) 0,35 (V) ✓ Zn(s) → Zn2+(aq) + 2e- 0,76 (V) ✓ X2+(aq) + Zn(s) ✓ X(s) +Zn2+(aq) 1,11 (V) ✓ X = Copper/Cu ✓ Accept: Cu/Cu2+ half reaction | |
(5)
8.4 Cu2+(aq) + Zn(s) ✓ → Zn2+(aq) + Cu(s) ✓ Bal. ✓
Accept:
- X2+(aq) + Zn(s) → Zn2+(aq) + X(s)
- Any metal identified in QUESTION 8.3 of which the ion has a +2 charge.
Notes
|
(3)
[15]
QUESTION 9
9.1 Electrolytic (cell) ✓ (1)
9.2 P ✓ (1)
9.3
9.3.1 Au(s) → Au3+(aq) + 3e- ✓✓
Ignore phases
| Notes Au3+ + 3e- ← Au (2) Au3+ + 3e- ⇌ Au (0/2) Au ⇌ Au3+ + 3e- (21) Au3+ + 3e → Au (0/2) |
(2)
9.3.2 (+)3 ✓ (1)
9.3.3 Electrical energy (is converted) to chemical energy. ✓(1)
9.3.4 Becomes smaller / thinner / eroded / decrease in mass. ✓(1)
9.4 ANY ONE
- Increase in value. ✓
- Protection against rust. (1)
9.5 ANY ONE
- Replace Au3+(aq) / electrolyte with Ag+(aq) / silver(I) solution / use a silver solution
- Replace P / anode / gold with Ag(s) / silver (1)
[9]
QUESTION 10
10.1
10.1.1 B/air ✓ & C/methane ✓ (2)
10.1.2 Nitric acid / HNO3 ✓ (1)
10.1.3 A / Sulphur / S ✓ (1)
10.1.4 2NH3(g) + H2SO4 ✓ → (NH4)2SO4 ✓ Bal. ✓ (3)
Notes
|
10.1.5 D / potassium chloride ✓ (1)
10.2
10.2.1
| OPTION 1: %P = 3 ✓x 22% 7 = 9,43% ∴m(P) = 9.43 × 2kg✓ 100 = 0,19 kg ✓ | OPTION 2: ∴m(P) 3 =✓(0,44) ✓( 22 x 2 = 0,44) 7 100 = 0,19 kg ✓ |
(3)
10.2.2
| OPTION 1 m(fertiliser) = 22 x 2 100 = 0,44 kg m(filler/bindstof) = 2 – 0,44✓ = 1,56 kg ✓ | OPTION 2 %filler = 100 – 22 ✓ = 78% m(filler) = 78 x 2 100 = 1,56 kg ✓ |
(3)
[14]
TOTAL: 150