PHYSICAL SCIENCES (CHEMISTRY)
MEMORANDUM QUESTION 1
[20]
QUESTION 2 2.1
2.1.1 A OR/OF D ✓ (1)
2.2
2.2.1
Marking criteria:
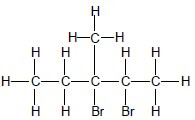
Five C atoms in longest chain. ✓ Two Br and one methyl substituents. ✓ Whole structure correct. ✓
Marking criteria:
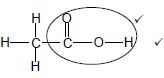
Whole structure correct: 2/2 Only functional group correct: Max 1/2 Accept -OH as condensed.
2.2.3
Marking criteria:
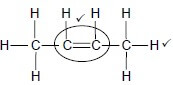
Whole structure correct: 2/2 Only functional group correct 1/2 IF: More than one functional group 0/2
2.3
2.3.1 Hydrogen (gas) ✓ (1)
[13]
QUESTION 3 3.1 Compounds with the same molecular formula ✓ but different structural formulae.✓ (2)
Structure: Intermolecular forces: Energy: More energy needed to overcome or break intermolecular forces / Van der Waals forces. ✓ From C to A : Structure : Intermolecular forces: Weaker / less intermolecular forces / Van der Waals forces / London forces / dispersion forces. ✓Energy: 3.4 A / 2,2-dimethylpropane ✓ → Lowest boiling point. ✓ (2) 5 H12 + 8O2 ✓ ⭢ 5CO2 + 6H2 O ✓ Bal ✓ (3)
Notes:
Reactants ✓ Products ✓ Balancing ✓ Ignore double arrows and phases. Marking rule 6.3.10. If condensed structural formulae used:Max.2/3
[11]
QUESTION 4 4.1
4.1.1 High temperature / heat / high energy / high pressure ✓ (1)
Accept: Condensed structural formula and structural formula.3 CH2 CH2 CH2 CHCH2
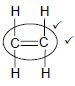
4.1.3 Alkenes ✓ (1)
4.2 X / C6H12 / Alkene / Hexene ✓ OPTION 1
X is an alkene / has a double bond / unsaturated. ✓ X can undergo addition. ✓ X will react without light / heat / is more reactive. ✓ OPTION 2
Butane is an alkane OR butane is saturated. ✓ Butane can only undergo substitution. ✓ Butane will only react in the presence of light / heat OR butane is less reactive. ✓ (4) 4.3
4.3.1 2-chloro✓butane ✓ (2)
Marking criteria:
Whole structure correct 2/2 Only functional group correct 1/2 IF: More than one functional group 0/2
4.3.4 Hydration ✓ (1)
[13]
QUESTION 5 5.1
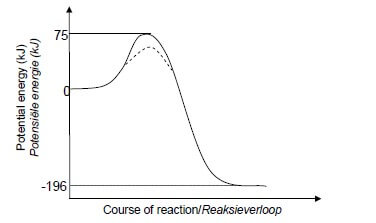
5.1.1 The minimum energy needed for a reaction to take place. ✓✓ OR
Marking criteria:
Shape of curve for exothermic reaction as shown.
✓
Energy of activated complex shown as 75 kJ in line with the peak.
✓
Energy of products shown as − 196 kJ below the zero.
✓
IF: Wrong shape, e.g. straight line.
0 /3
5.1.3 Marking criteria
Dotted line (---) on graph in QUESTION 5.1.2 showing lower energy for activated complex. ✓ Dotted curve starts at/above energy of reactants and ends at/above energy of products on the inside of the original curve. ✓ (2) Note:
5.1.4
A catalyst provides an alternative pathway of lower activation energy. ✓ More molecules have sufficient / enough (kinetic) energy. ✓ OR More molecules have kinetic energy equal to or greater than the activation energy. More effective collisions per unit time / second. ✓ Rate / frequency of effective collisions increases. (3) 5.2
5.2 .1 Ave rate. tempo = ΔV 52 - 16 ✓ 3 .s-1 )✓ (3)
Accept:
Volume range: 16 to 17 cm3 Answer range: 1,167 to 1,2 dm3 ∙s-1
5.2.2 (4)
Marking criteria :
V(O2 ) = 60 dm3 AND divide volume by 24 ✓ Use ratio: n(H2 O2 ) = 2n(O2 ) = 1:2 ✓ Use 34 g∙mol-1 in n = m or in ratio calculation. ✓ Final answer: 170 g ✓ OPTION 1 2 ) = V M 60 ✓2 O2 ) = 2n(O2 ) 2 O2 ) = m M m
OPTION 2 3 : 1 mol 3 : 2,5 mol ✓ 2 O2 ) = 2n(O2 )
OPTION 3
n(O2 ) = V M 60 ✓ n(O2 ) = m M∴2,5 = m 32 ∴m = 80 g 2 O2 .......32 g O2 x g H2 O2 ................ 80 g O2 m(H2 O2 ) = 170 g ✓
5.2.3 Equal to (1)
5.3
5.3.1 Q ✓ (1)
[20]
QUESTION 6 6.1 The stage in a chemical reaction when the rate of forward reaction equals the rate of reverse reaction. ✓✓ (2 marks or no marks) OR The state where the concentrations / quantities of reactants and products remain constant. (2)
6.2.1 Remains the same✓ (1)
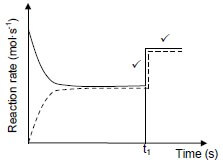
When the temperature is increased the reaction that will oppose this increase / decrease the temperature will be favoured. ✓ The forward reaction is exothermic. An increase in temperature favours the endothermic reaction. ✓ The reverse reaction is favoured. ✓ (4) 6.3
Marking criteria:
Vertical parallel lines show a sudden increase in rate of both forward and reverse reactions. ✓ Horisontal parallel lines showing a constant higher rate for both forward and reverse catalysed reactions after time t1. ✓
6.4 CALCULATIONS USING NUMBER OF MOLES
Marking criteria:
Use M(PbS) = 239 g∙mol-1 in n = m or in ratio calculation ✓ Use ratio: n(H2 S)equil = n(PbS) ✓ n(H2 S)formed = n(H2 S)equilibrium ✓ USING ratio: H2 : H2 S = 1 : 1 ✓ n(H2 )equilibrium = n(H2 )initia – n(H2 )formed ✓ Divide equilibrium n(H2 S) & n(H2 ) by 2 dm3 . ✓ Correct Kc expression ✓ Substitution of concentrations into Kc expression. ✓ Final answer: 0,07 ✓
OPTION 1 m = 2,39 = 0,01 mol2 S)equilibrium = n(PbS) ✓ = 0,01 mol
H2 H2 S Initial quantity (mol) 0,16 0 Chnage (mol) 0,01 0,01✔ ratio✔ Quantity at equilibrium (mol) 0,15 ✔ 0,01 Equlibrium concentration (mol.dm-3 ) 0,075 0,005 divide by 2✔
Kc = [H2 S ] ✔2 ] 0,005 ✔
No Kc expression, correct substitution: Max : 8/9 Wrong Kc expression: Max : 6/9 IF: [S] = 1 in Kc = [H2 S ] 2 ][S ]c expression, but continue marking substitution and answer
OPTION 2 m = 2,39 = 0,01 mol2 S)reacted = n(PbS) ✓ = 0,01 mol 2 S)equilibrium n(H2 S)formed = n(H2 S)equilibrium – n(H2 S)initial = 0,01 – 0 ✓ 2 )reacted = n(H2 S)formed ✓ = 0,01 mol 2 )equilibrium = n(H2 )initial - n(H2 )reacted = 0,16 - 0,01 ✓ 2 ) = n c(H2 S) = n 0,15 = 0,01 -3 = 0,005 mol.dm-3
Kc = [H2 S ] ✔2 ] 0,005 ✔
No Kc expression, correct substitution: Max : 8/9 Wrong Kc expression: Max : 6/9 IF: [S] = 1 in Kc = [H2 S ] 2 ][S]c expression, but continue marking substitution and answer
OPTION 3
H2 H2 S Initial quantity (mol) 0,16 0 Change (mol) x x✔ ratio✔ Quantity at equilibrium (mol) 0,16 - x ✔ x Equlibrium concentration (mol.dm-3 ) 0,16 - x x divide by 2✔
n(PbS) = m = 2,39 = 0,01 mol2 S)equilibrium = n(PbS) ✔ ∴ x = 0,01 mol2 ]equilibrium = 0,16 - 0,01 = 0,075 mol.dm-3 2 S]equilibrium = 0,01 = 0,005 mol.dm-3
Kc = [H2 S ] ✔2 ] 0,005 ✔
No Kc expression, correct substitution: Max : 8/9 Wrong Kc expression: Max : 6/9 IF: [S] = 1 in Kc = [H2 S ] 2 ] [S]c expression, but continue marking substitution and answer
CALCULATIONS USING CONCENTRATION
Marking criteria:
Use M(PbS) = 239 g∙mol-1 in n = m or in ratio calculation ✓ Use ratio: n(H2 S)equil = n(PbS) ✓ Divide equilibrium n(H2 S)equil & n(H2 )initial by 2 dm3 . ✓ (H2 S) formed = (H2 S)equal ✓ USING ratio: H2 :H2 S =1 :1 [H2 ]equilibrium = [H2 ]initial – [H2 ]formed ✓ Correct Kc expression ✓ Substitution of concentrations into Kc expression. ✓ Final answer: 0,07 ✓ Note: If not rounded: 0,067
OPTION 4
n(PbS) = m = 2,39 = 0,01 mol2 S)equilibrium = n(PbS) ✓ = 0,01 mol
H2 H2 S Initia concentration ( mol.dm-3 ) 0,16 / 2 = 0,08 0 Change in concentration ( mol.dm-3 ) 0,05 0,05✔ ratio✔ Equlibrium concentration (mol.dm-3 ) 0,075 0,005 divide by 2✔
Kc = [H2 S ] ✔2 ] 0,005 ✔
No Kc expression, correct substitution: Max : 8/9 Wrong Kc expression: Max : 6/9 IF: [S] = 1 in Kc = [H2 S ] 2 ][S ]c expression, but continue marking substitution and answer
OPTION 5
n(PbS) = m = 2,39 = 0,01 mol2 S)equilibrium = n(PbS) ✓ = 0,01 mol 2 S]equilibrium = n 0.01 -3 2 ]initial = n 0.16 -32 S] formed = [H2 S] equilibrium - [H2S] initial mol.dm-3 [H2 ]reacted = [H2 S]formed = 0,005 mol[ H2 ]equilibrium= [H2 ]initial- [H2 ]reacted = 0,008 - 0,005= 0,075 mol
Kc = [H2 S ] ✔2 ] 0,005 ✔
No Kc expression, correct substitution: Max : 8/9 Wrong Kc expression: Max : 6/9 IF: [S] = 1 in Kc = [H2 S ] 2 ][S ]c expression, but continue marking substitution and answer
(9) [18]
QUESTION 7 7.1
7.1.1 Hydrolysis is the reaction (of a salt) with water. ✓✓ (2 or 0)
7.1.2 Smaller than (7) ✓ 4 + + H2 O ✓ → NH3 + H3 O+ ✓ 4 Cℓ + H2 O → NH3 + H3 O+ + Cℓ- 4 + → NH3 + H+ ((3)
Note:
Mark equation independently of first answer. If incorrect balancing: Max 2/3
7.2
Marking criteria for equation:
Reactants ✓ Products ✓ Ignore double arrows and phases. Marking rule 6.3.10
7.2.1 (2)
Marking guidelines:
Substitution of 98 g∙mol-1 . ✓ Final answer: 0,08 mol ✓ Note: OPTION 1
n = m = 7,35 = 0,08 mol
OPTION 2
OPTION 3
n = m = 7,35 -3
7.2.2 POSITIVE MARKING FROM QUESTION 7.2.1.
OPTION 1
pH = −log[H3 O+ ] ✓ 3 O+ ] 3 O+ ] = 0,05 mol∙dm-3 [H2 SO4 ] = ½[H3 O+ ] -3 (0,03)
n(H2 SO4 )ex = cV ✓ 2 SO4 )react = 0,075 – 0,0125 ✓ [the highlighted part is from Q7.2.1] 2 SO4 )
OR EITHER⇒⇒⇒ ⇓ ⇓ ⇓
n(NaOH) = m = 0,125 = m
m = 5 g ✓ (4,8 g)
Marking guidelines:
Formula: pH = −log[H3 O+ ] ✓ Substitution of 1,3 ✓ Use [H2 SO4 ] : [H3 O+ ] = 1 : 2 ✓ Formula: c = n ✓ Multiply by 0,5 dm3 ✓ Subtract ninitial – nexcess ✓ Use n(NaOH) : n(H2 SO4 ) = 2:1 ✓ Substitution of 40 g∙mol-1 ✓ Final answer: m = 5 g ✓
1 mol : 40 g ✓
OPTION 2
pH = −log[H3 O+ ] ✓ 3 O+ ] 3 O+ ] = 0,05 mol∙dm-3 n(H3 O+ )ex = cV ✓ 3 O+ )in = 2n(H2 SO4 ) [the highlighted part is from Q7.2.1] 3 O+ )react = 0,15 – 0,025 ✓ + ) ✓
OR EITHER⇒⇒⇒ ⇓ ⇓ ⇓
n(NaOH) = m = 0,125 = m
m = 5 g ✓ (5,2 g)
Marking guidelines:
Formula: pH = −log[H3 O+ ] ✓ Substitution of 1,3 ✓ Formula/Formule: c = n ✓ Multiply by 0,5 dm3 ✓ Use n(H2SO4) : n(H3 O+ ) = 1 : 2 ✓ Subtract ninitial – nexcess ✓ Use n(H3 O+ ) : n(NaOH) = 1 : 1 ✓ Substitution of 40 g∙mol-1 ✓ Final answer: m = 5 g ✓
1 mol : 40 g ✓
OPTION 3 2 S]ein = n = 0,075 [the highlighted part is from Q7.2.1] 3 (0,16) 3 O+]in = 2[H2 SO4 ] = 0,3 mol∙dm-3 (0,32) 3 O+ ] ✓ 3 O+ ] 3 O+ ] = 0,05 mol∙dm-3 3 O+ ]react = 0,3 – 0,05✓ -3 (0,27) 2 SO4 ]react = ½[H3 O+ ]
Marking guidelines
Formula: c = n ✓ Divide by 0,5 dm3 ✓ Use [H3 O+ ] : [H2 SO4 ] = 2:1 ✓ Formula: pH = −log[H3 O+ ] ✓ Substitution of 1,3 ✓ Subtract [H3 O+ ]initial – [H3 O+ ]excess ✓ Use n(NaOH) : n(H2 SO4 ) = 2:1 ✓ Use [H2 SO4 ] : [NaOH] = 1 : 2 ✓ Substitution of 40 g∙mol-1 ✓ Final answer: m = 5 g ✓ n(H2 SO4 )react = cV 2 SO4 )
n(NaOH) = m m
[H2 SO4 ] : [NaOH] 1 : 2
m = cMV
(9) [16]
QUESTION 8
8.1
8.1.1 AgNO3 / Silver nitrate ✓ (1) 2+ + 2e- ✓✓ (2)
Marking guidelines:
Ni ⇌ Ni2+ + 2e- ½ Ni2+ + 2e- ⇌ Ni 0 /2 2+ + 2e- → Ni ½ Ni2+ + 2e- ← Ni 0 /2 Ignore if charge omitted on electron. If charge (+) omitted on Ni2+ : Max: 21 Example: Ni → Ni2 + 2e- ✓
8.1.3 Ni + 2Ag+ ✓ → Ni2+ + 2Ag ✓ Bal ✓ 3 → Ni(NO3 )2 + 2Ag (3)
Notes:
Reactants ✓ Products ✓ Balancing: ✓ Ignore double arrows. Marking rule 6.3.10/
8.2
8.2.1 Ni ✓ - Ni is a stronger reducing agent. / Ni has a higher reducing ability. / Ni is the anode. / Ni loses electrons. / Ni is oxidised. ✓ (2) ✓ ✓ ✓
8.2.2 Ni (s) | Ni2+ (aq) || Ag+ (aq) | Ag(s) OR 2+ (1 mol∙dm-3 ) || Ag+ (1 mol∙dm-3 ) | Ag(s) Accept: Ni | Ni2+ || Ag+ | Ag (3)
8.2.3 (4)
OPTION 1: Eθ cel l = Eθ reduction −Eθ oxidation ✓
Notes
Accept any other correct formula from the data sheet Any other formula using unconventional abbreviations, e.g. E°cell = E°OA - E°RA followed by correct substitutions: ¾ OPTION 2 + + e- ⭢ Ag✓ Eθ = 0,80 V ✓ 2+ + 2e- Eθ = +0,27 V ✓ + + Ni ⭢ Ag + Ni2+ Eθ = +1,07 V ✓
8.2.4 Increases ✓ (1)
[16]
QUESTION 9
9.3
9.3.1 Chlorine (gas) / Cℓ2 ✓ (1) 2 ✓ (1) 2 O(ℓ) + 2e- ⭢ H2 (g) + 2OH- (aq) ✓✓ (2)
Notes 2 (g) + 2OH- (aq) ← 2H2 O(ℓ) + 2e- 2/2 2H2 O(ℓ) + 2e- ⇌ H2 (g) + 2OH- (aq) 1/2 2 (g) + 2OH- (aq) ⇌ 2H2 O(ℓ) + 2e- 0/2 2H2 O(ℓ) + 2e- ← H2 (g) + 2OH- (aq) 0/2
9.4 Basic ✓ - OR Alkaline − (ions) / NaOH / Strong base forms.✓ (2)
[9]
QUESTION 10
10.1.1 Haber (process) ✓ (1) 2 ✓ (1) 3 ✓ (1) 3 + H2 SO4 ✓ ⭢ H2 S2 O7 ✓ Bal. ✓ (3)
Notes
Reactants ✓ Products ✓ Balancing ✓ Ignore ⇌ and phases Marking rule 6.3.10
10.1.5 H2 SO4 ✓ + 2NH3 ✓ ⭢ (NH4 )2 SO4 ✓ Bal. ✓ (4)
Notes
Reactants ✓✓ Products ✓ Balancing ✓ Ignorer ⇌ and phases Marking rule 6.3.10
10.2 (4)
Marking guidelines:
Calculate the mass of fertiliser. Add %N and %P OR mass N and mass P. Subtraction: 100 – (%N + %P) Final answer: 8:1:5
OPTION 1:
m(fertiliser) = 36 /100 x 20 4,11 x 100 0,51 x 100
OPTION 2:
m(fertiliser) = 36 /100 x 20 ✓
OPTION 3
%N =4,11 x 100 = 20,55% ✓0,51 x 100 = 2,55%
20,55 : 2,55 : 12,9
[14]
 (3)
(3) (2)
(2) (2)
(2) (2)
(2) (3)
(3)  (2)
(2)