PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS NOVEMBER 2021
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupQUESTION 1
1.1 D ✔✔ (2)
1.2 D ✔✔ (2)
1.3 A ✔✔ (2)
1.4 B ✔✔ (2)
1.5 D ✔✔ (2)
1.6 D ✔✔ (2)
1.7 C ✔✔ (2)
1.8 B ✔✔ (2)
1.9 A ✔✔ (2)
1.10 B ✔✔ (2)
[20]
QUESTION 2
2.1 A compound that contains a double bond/multiple bond/does NOT contain only single bonds (between C atoms). ✔✔ (2 or 0) (2)
2.2
2.2.1 B / E ✔ (1)
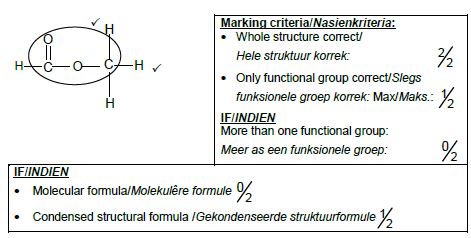
2.2.2 Carbonyl (group bonded to two C atoms) ✔
ACCEPT
Ketone (1)
2.2.3 F ✔✔ (2)
2.2.4 2,5-dichloro-3-methylhexane
Marking criteria:
|
(3)
2.2.5 CnH2n ✔ (1)
2.3 Compounds with the same molecular formula, ✔ but different functional groups/homologous series. ✔(2)
2.4
2.4.1 Carboxylic acids✔ (1)
2.4.2
(2)
2.5
2.5.1 Ethanol/✔ (1)
2.5.2 E ✔ ACCEPT : C2H4 (1)
2.5.3 (Concentrated) sulphuric acid/H2SO4/(concentrated) phosphoric acid/H3PO4 ✔ (1)
[18]
QUESTION 3
3.1
| Marking criteria If any one of the underlined key phrases in the correct context is omitted, deduct 1 mark. |
The temperature at which solid and liquid phases are in equilibrium. ✔✔(2)
3.2
Marking criteria
|
As the chain length/number of C atoms/molecular mass/surface area/strength of the intermolecular forces ✔ increases, the melting points increase. ✔
OR
- As the chain length/ number of C atoms/molecular mass/surface area/strength of the intermolecular forces ✔ decreases, the melting points decrease. ✔(2)
3.3
London forces ✔
Londonkragte
ACCEPT
Dispersion forces/induced dipole forces (1)
3.4
3.4.1 Liquid✔ (1)
3.4.2 Solid✔ (1)
3.5
3.5.1 Equal to ✔
Same molecular formula/Isomers/same number and types of atoms/same number of C and H atoms ✔ (2)
3.5.2 Lower than ✔ (1)
3.5.3
Marking criteria:
|
2,2-dimethylbutane:
- Structure:
More branched/more compact/more spherical/smaller surface area (over which intermolecular forces act).✔ - Intermolecular forces:
Weaker/less intermolecular forces/Van der Waals forces/London forces/dispersion forces. ✔ - Energy:
Lesser energy needed to overcome or break intermolecular forces/Van der Waals forces. ✔
OR
Hexane - Structure:
Longer chain length/unbranched/less compact/less spherical/larger surface area (over which intermolecular forces act). ✔ - Intermolecular forces:
Stronger/more intermolecular forces/Van der Waals forces/London forces/dispersion forces. ✔ - Energy:
More energy needed to overcome or break intermolecular forces/Van der Waals forces. ✔
QUESTION 4
4.1
4.1.1 Substitution/Hydrolysis ✔
Substitusie/Hidrolise (1)
4.1.2 Primary (alcohol) ✔
ANY ONE:
- The C atom of the functional group is the terminal C atom.
- The C-atom bonded to the hydroxyl/-OH is bonded to (only) one other Catom. ✔
- The hydroxyl/-OH is bonded to a C-atom which is bonded to two hydrogen atoms.
- The hydroxyl/-OH is bonded to a primary C atom/terminal C atom/first C atom. ✔(2)
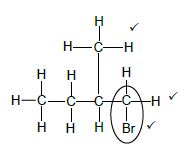
4.1.3
Marking criteria:
- Four C atoms in longest chain. ✔
- One methyl substituent on C2. ✔
- Bromo substituent on C1. ✔
IF - Any error e.g. omission of H atoms, condensed or semi structural formula/Enige fout bv.
Max:2/3 (3)
4.1.4 Elimination/dehydrohalogenation/dehydrobromination ✔(1)
4.1.5 Alkenes/Alkene ✔ (1)
4.1.6 Addition/Addisie ✔ (1)
4.1.7 2-bromo-2-methyl✔butane ✔
2-bromo-2-metiel✔butaan ✔ (2)
4.2
NOTE
- Penalise only once for the use of structural formulae or molecular formulae.
4.2.1 Marking criteria:
- Correct condensed structure for but-2-ene. ✔
- React but-2-ene with H2/H — H. ✔
- Indicate the catalyst Pt/Ni/Pd on arrow/at the equation. ✔
- Correct condensed formula for butane as product. ✔
IF: Any additional products or reactants - minus 1 mark
ACCEPT
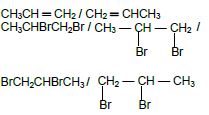
As reactant: CH3(CH)2CH3 / CH3CH ═ CHCH3 / CH3 — CH ═ CH — CH3
As product: CH3(CH2)2CH3 / CH3 — CH2 — CH2 — CH3 /CH3 — (CH2)2 — CH3 (4)
4.2.2 Elimination/Cracking (1)
4.2.3 Propene/1-propene/prop-1-ene ✔✔ (2)
4.2.4
Marking criteria:
- Correct condensed formula for propene as reactant. ✔
- React (propene) with Br2/Br — Br ✔
- Correct condensed formula for 1,2-dibromopropane as product. ✔
IF: Any additional products or reactants - minus 1 mark
CH3CHCH2 ✔ + Br2 ✔ → CH3CHBrCH2Br ✔
ACCEPT :
As reactant CH3CH ═ CH2 / CH2 ═ CHCH3
As product CH3CHBrCH2Br / / BrCH2CHBrCH3 (3)
[21]
QUESTION 5
5.1 NOTE
Give the mark for per unit time only if in context of reaction rate.
ANY ONE
- Change in concentration ✔ of products/reactants per (unit) time. ✔
- Change in amount/number of moles/volume/mass of products or reactants per (unit) time.
- Amount/number of moles/volume/mass of products formed/reactants used per (unit) time.
- Rate of change in concentration/amount of moles/number of moles/volume/ mass. ✔✔ (2 or 0)
(2)
5.2 Reaction rate decreases./Concentration of HCℓ decreases./Concentration of reactant decreases./Reactants are used up/Mass of CaCO3 decreases or is used up. ✔(1)
5.3
5.3.1 Exothermic/Eksotermies ✔ (1)
5.3.2
- Gradient increases/becomes steeper. / Curve becomes steeper. ✔
- Reaction rate increases/More (or larger volume) of CO2 is produced per unit time. ✔
- Temperature increases./Energy is released/Average kinetic energy of the molecules increases. ✔(3)
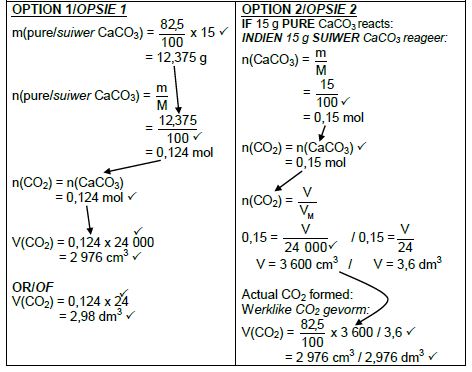
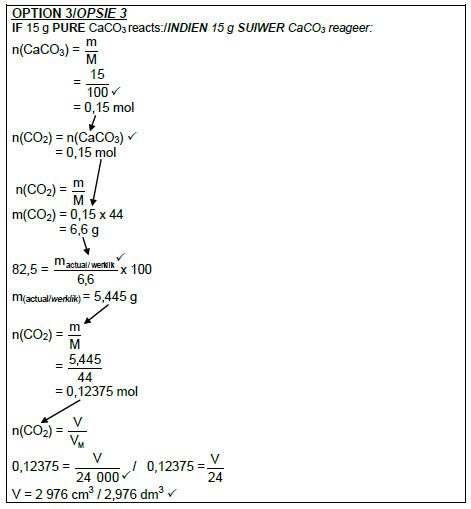
5.4 Marking criteria
- m(pure CaCO3) = 82,5/ 100 x 15 ✔ / V(CO2) = 82,5/ 100 x V(CO2) from15 g CaCO3
- Divide by 100 g∙mol-1. ✔
- Use mol ratio: n(CO2) = n(CaCO3). ✔
- Multiply n(CO2) by 24 000 cm3/24 dm3. ✔
- Final answer: 2 976 cm3 ✔
- Range: 2880 to 2970 cm3 / 2,88 to 2,97 dm3
(5)
5.5 Increases/Toeneem ✔ (1)
5.6 More (CaCO3) particles with correct orientation/exposed./ Greater (exposed) surface area. ✔
More effective collisions per unit time./Higher frequency of effective collisions. ✔
NOTE
- If explanation in terms of CONCENTRATION: No mark for bullet 1.
- Bullets are marked independently. (2)
[15]
QUESTION 6
6.1 (The stage in a chemical reaction when the) rate of forward reaction equals the rate of reverse reaction. ✔✔ (2 or 0)
OR
(The stage in a chemical reaction when the) concentrations of reactants and products remain constant. (2 or 0)
(2)
6.2
6.2.1 Negative/Negatief ✔ (1)
6.2.2
- Increase in temperature favours an endothermic reaction. Accept: Decrease in temperature favours an exothermic. ✔
- Reverse reaction is favoured./Concentration of reactants increases./ Concentration of products decreases. ✔
- (Forward) reaction is exothermic.
Accept: Reverse reaction is endothermic. ✔
(3)
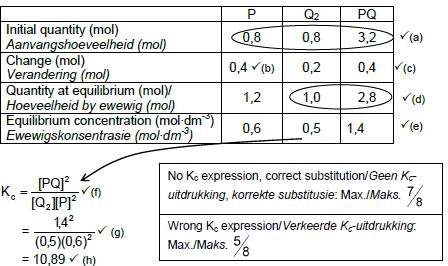
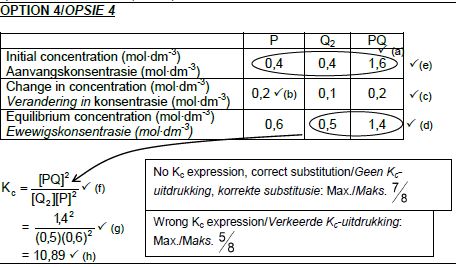
6.2.3 CALCULATIONS USING NUMBER OF MOLES
Marking criteria:
- Initial n(P) and n(Q2) and n(PQ) from table. ✔
- Change in n(P) = equilibrium n(P) – initial n(P).✔
- USING ratio: P : Q2 : PQ = 2 : 1 : 2 ✔
- Equilibrium n(Q2) = initial n(Q2) + change in n(Q2)
Equilibrium n(PQ) = initial n(PQ) - change in n(PQ) - Divide equilibrium amounts of P and Q2 and PQ by 2 dm3. ✔
- Correct Kc expression (formulae in square brackets). ✔
- Substitution of equilibrium concentrations into Kc expression. ✔
- Final answer: 10,889 ✔
(3)
OPTION 1
OPTION 2
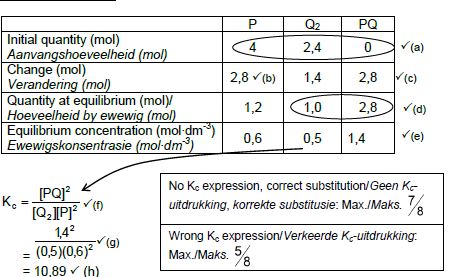
CALCULATIONS USING NUMBER OF MOLES
Marking criteria:
- Initial n(P) = 4 mol and n(Q2) = 2,4 mol and n(PQ) = 0 ✔
- Change in n(P) = equilibrium n(P) – initial n(P) = 2,8 mol.✔
- USING ratio: P : Q2 : PQ = 2 : 1 : 2 ✔
- Equilibrium n(Q2) = initial n(Q2) + change in n(Q2)
Equilibrium n(PQ) = initial n(PQ) - change in n(PQ) - Divide equilibrium amounts of P and Q2 and PQ by 2 dm3. ✔
- Correct Kc expression (formulae in square brackets). ✔
- Substitution of equilibrium concentrations into Kc expression. ✔
- Final answer: 10,89 / 10,889 ✔
OPTION 3
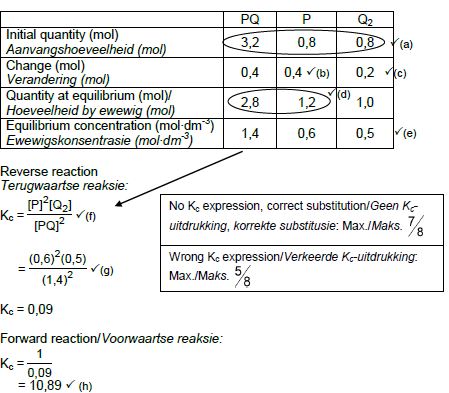
CALCULATIONS USING CONCENTRATION
Marking criteria:
- Initial c(P) and c(Q2) and c(PQ) from table. ✔
- Change in c(P) = equilibrium c(P) – initial c(P). ✔
- USING ratio: P : Q2 : PQ = 2 : 1 : 2 ✔
- Equilibrium c(Q2) = initial c(Q2) + change in c(Q2)
Equilibrium c(PQ) = initial c(PQ) - change in c(PQ) - Divide initial amounts of P and Q2 and PQ by 2 dm3. ✔
- Correct Kc expression (formulae in square brackets). ✔
- Substitution of equilibrium concentrations into Kc expression. ✔
- Final answer: 10,89 / 10,889 ✔
(8)
6.2.4 Remains the same/Bly dieselfde ✔
Only temperature can change Kc./Temperature remains constant. ✔(2)
6.3
6.3.1 Increases✔ (1)
6.3.2 Decreases✔ (1)
[18]
QUESTION 7
7.1
7.1.1 (It is a) proton/H3O+ (ion)/H+ (ion) donor. ✔✔ (2)
7.1.2 HSO-4 /hydrogen sulphate ion/waterstofsulfaatioon ✔
ANY ONE:
- It acts as base in reaction I and as acid in reaction II. ✔
- Acts as acid and base.(2)
7.1.3 HSO4- /Reaction (solution) II ✔
Smaller Ka value/weaker acid ✔
Lower ion concentration/Incompletely ionised. ✔
(3)
7.2
7.2.1
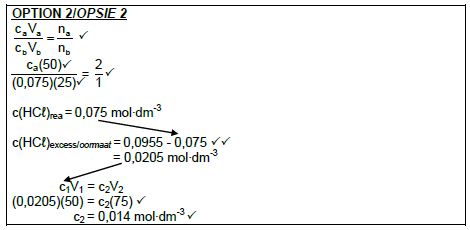
| OPTION 1 pH = -log[H3O+] ✔ 1,02 ✔= -log[H3O+] [H3O+] = 0,0955 mol∙dm-3 ✔ Therefore/Dus [HCℓ = 0,0955 mol∙dm-3 (0 0 6/0 1 mol∙dm-3) | OPTION 2 pH = -log[H3O+] [H3O+] = 10-pH = 10-1,02 ✔ = 0,0955 mol∙dm-3 ✔ Therefore/Dus [HCℓ = 0,0955 mol∙dm-3 (0 0 6/0 1 mol∙dm-3) |
(3)
✔Any one
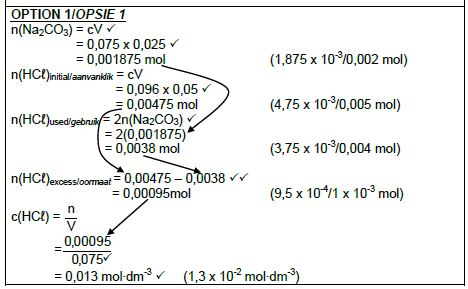
7.2.2 POSITIVE MARKING FROM 7.2.1
Marking citeria:
- Formula: C = n/V / cₐvₐ = nₐ/nb
CbVb - Calculate n(Na2CO3): 0,075 x 0,025 ✔
- Calculate n(HCℓ): 0,0955 x 0,05 / 0,096 x 0,05 ✔
- Use ratios: n(HCℓ) = 2n(Na2CO3) ✔
- n(HCℓ)excess = n(HCℓ)initial – n(HCℓ)used = 0,00475 – 0,0038 ✔✔
- Substitute 0,075 dm3 in c = n/v
- Final answer: 0,013 mol∙dm-3 ✔ (1,3 x 10-2 mol∙dm-3)
Range: 0,01 to 0,02 mol∙dm-3
(8)
[18]
QUESTION 8
8.1 Chemical (energy) to electrical (energy) ✔ (1)
8.2 Marking criteria:
- Any formula: c = m /c = n/n = m/M
MV V - Substitute 1 mol∙dm-3.✔
- Substitute 170 g∙mol-1 [or 108 + 14 + 3(16)] and 0,15 dm3 in correct formulae. ✔
- Final answer: 25,50 g ✔
8.3 ANY ONE:
- A substance that loses/donates electrons. ✔✔
- A substance that is oxidised.
- A substance whose oxidation number increases. (2)
8.4
8.4.1 Copper✔ (1)
8.4.2 Marking criteria/
- Reactants ✔ Products ✔ Balancing ✔
- Ignore double arrows.
- Ignore phase
- Marking rule 6.3.10.
Cu(s) + 2Ag+(aq) ✔ → Cu2+(aq) + 2Ag(s) ✔ Bal ✔
ACCEPT :
Cu(s) + 2AgNO3(aq) ✔ → Cu(NO3)2(aq) + 2Ag(s) ✔ Bal ✔
NOTE/LET WEL - IF electrons are not cancelled – minus 1 mark
(3)
8.5 OPTION 1
Eθcell = Eθreduction - Eθoxidation ✔
= 0,80 ✔ – (0,34) ✔
= 0,46 V ✔
Notes
- Accept any other correct formula from the data sheet.
- Any other formula using unconventional
abbreviations, e.g. E°cell = E°OA - E°RA followed by correct substitutions:
OPTION 2
2Ag+ + 2e- → 2Ag Eθ = 0,80 V ✔
Cu → Cu2+ + 2e- Eθ = - 0,34 V ✔
2Ag+ + Cu → 2Ag + Cu2+ Eθ= +0,46 V ✔
(4)
8.6 Decreases (1)
[16]
QUESTION 9
9.1 ANY ONE: (2 or 0)
- A substance whose (aqueous) solution contains ions. ✔✔
- Substance that dissolves in water to give a solution that conducts electricity.
- A substance that forms ions in water / when melted.
- A solution that conducts electricity through the movement of ions.
(2)
9.2 Anode ✔
- Chromium is oxidised./Oxidation takes place (at the anode)./Chromium (it) loses electrons./Mass decreases./Cr → Cr3+ + 3e- ✔
NOTE/LET WEL:
If half-reaction is used, it must be correct/Indien halfreaksie gebruik word: Cr → Cr3+ + 3e- (2)
9.3 Cr3+(aq) + 3e- → Cr(s) ✔✔
Ignore phases.
Marking guidelines
- Cr3+ + 3e- ⇌ Cr 1/2
- Cr ⇌ Cr3+ + 3e- 0/2
- Cr ← Cr3+ + 3e- 2/2
- Cr → Cr3+ + 3e- 0/2
- Ignore if charge omitted on electron.
- If charge (+) omitted on Cr3+Max: 1/2
Example/Voorbeeld: Cr3 + 3e- → Cr ✔
(2)
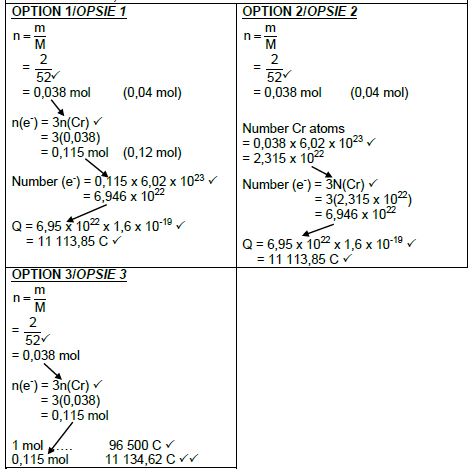
9.4 Marking criteria:
- Substitute 52 g∙mol-1 in m/M/ratio ✔
- Use mol ratio: n(electrons): n(Cr) = 3 : 1. ✔
- Number of electrons = n x 6,02 x 1023/No of Cr atoms = n x 6,02 x 1023/ratio. ✔
- Total charge = number of electrons x 1,6 x 10-19/ratio. ✔
- Final answer: 11 113,85 C ✔
Range: 11 076,8 to 11 580 C
[11]
TOTAL: 150