Physical Sciences Paper 2 Questions - Grade 12 June 2021 Exemplars
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your name and surname in the appropriate space on the ANSWER BOOK.
- This question paper consists of SEVEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 The general formula for ALKANES is …
- CnH2n.
- CnH2n-1.
- CnH2n-2.
- CnH2n+2. (2)
1.2 The following graph shows the relationship between the number of carbon atoms in a straight chain molecules of alkanes, alcohols and aldehydes.
Curves P, Q and R is obtained.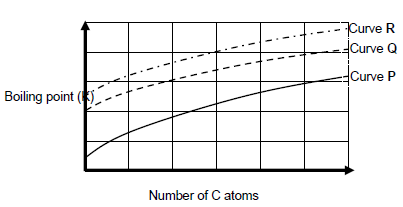
Which ONE of the following CORRECTLY describes the homologous series against the curve?
Curve P | Curve Q | Curve R | ||
A | Alcohols | Aldehydes | Alkanes | |
B | Aldehydes | Alcohols | Alkanes | |
C | Alcohols | Alkanes | Aldehyde | |
D | Alkanes | Aldehydes | Alcohols |
(2)
1.3 An alkene reacts with an EXCESS amount of water in the presence of an acid catalyst to produce compound P as shown in the equation below.
ALKENE + H2O → compound P
Compound P is a(n) …
- alcohol.
- alkane.
- haloalkane.
- carboxylic acid. (2)
1.4 Activation energy of a chemical reaction is defined as:
- Net energy released
- Net energy absorbed
- Minimum energy needed to start the reaction
- Maximum energy needed to start the reaction (2)
1.5 Consider the potential energy diagram for a reversible hypothetical reaction represented by the balanced equation below.
X2(g) ⇌ 2X(g)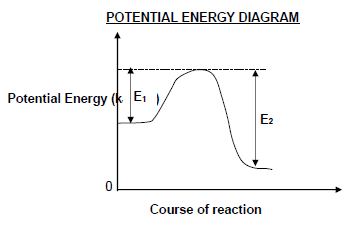
E1 and E2 are the activation energies for the forward and reverse reactions respectively. The difference (E2 – E1) is equal to …
- energy of the product.
- ΔH for the forward reaction.
- ΔH for the reverse reaction.
- energy of the activated complex. (2)
1.6 A chemical reaction reaches chemical equilibrium in a closed system. At equilibrium concentration of products and reactants remains constant because the rate of the forward reaction is …
- zero.
- higher than the rate of the reverse reaction.
- lower than the rate of the reverse reaction.
- equal to the rate of the reverse reaction. (2)
1.7 Consider the following reaction at equilibrium in a closed system.
Cu(H2O)62+ (aq) + 4 Cℓ- (aq) ⇋ CuCℓ42- (aq) + 6 H2O (ℓ) ΔH > 0
BLUE GREEN
Which ONE of the following changes to the equilibrium mixture above will ensure a COLOUR change from GREEN to BLUE?
- Increase in pressure
- Addition of silver nitrate
- Increase in temperature
- Addition of hydrochloric acid (2)
1.8 The endpoint in a titration is the exact point where …
- neutralisation occurs.
- the indicator changes colour.
- equal masses of base and acid have reacted.
- equal number of moles of acid and base have reacted. (2)
1.9 In the table below the ionisation constant values for bases, Kb at 25 °C are given.
Which ONE of the following bases is the STRONGEST?
BASE | Kb at 25 °C | |
A | SO42- | 8,3 x 10 -13 |
B | PO43- | 5,9 x 10-3 |
C | HCO3- | 2,4 x 10 -8 |
D | CH3COO- | 5,6 x 10 -10 |
(2)
1.10 Water undergoes auto-ionisation according to the balanced equation:
H2O(ℓ) + H2O(ℓ) ⇋ H3O+ (aq) + OH- (aq) ΔH > 0
The ionisation constant Kw for water is 1,00 x 10 -14 at 25 °C
Which ONE of the following is TRUE when the temperature of water in a beaker is increased from 25 °C to 30 °C?
- Kw remains the same and the water becomes acidic
- Kw remains the same and the water remains neutral
- Kw increases and the water remains neutral
- Kw decreases and the water remains neutral (2)
[20]
QUESTION 2 (Start on a new page.)
2.1 The compound P, shown below, belongs to alkynes which is a group of organic compounds with the same general formula and functional group.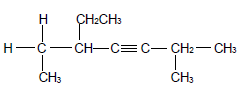
2.1.1 Write down a general term for the underlined phrase. (1)
2.1.2 Is compound P a SATURATED or UNSATURATED compound? Give a reason for the answer. (2)
For compound P write down the:
2.1.3 Empirical formula (1)
2.1.4 IUPAC name (3)
2.2 Consider compounds A and B given below.
- propan-1-ol
- HCOOH
2.2.1 Write down the STRUCTURAL formula of compound A. (2)
Compounds A and B are heated together in the presence of a catalyst in a test tube to produce an ESTER.
2.2.2 Describe how the mixture of A and B in the test tube is heated. (2)
For the reaction between compounds A and B write down the:
2.2.3 Name of the reaction taking place (1)
2.2.4 Formula of the catalyst used (1)
2.2.5 STRUCTURAL formula of the ester that is produced (2)
2.3 Haloalkanes, compounds C, D and E are structural isomers.
Compounds C and D are shown below and compound E is not shown:
C: CH3CH2CH2CH2Br D: (CH3)3CBr
2.3.1 Define the term structural isomer. (2)
2.3.2 Give a reason why compound C is classified as a PRIMARY haloalkane. (2)
2.3.3 Write down the IUPAC name of compound E, the POSITIONAL isomer of compound D. (3)
[22]
QUESTION 3 (Start on a new page.)
A group of learners investigated the effect of intermolecular forces on the boiling points of compounds.
They tabulated their results below:
Compounds Boiling point (°C) A Propan-1-ol 97 B Butan-1-ol 117,7 C Pentan-1-ol 138 |
3.1 Define the term boiling point. (2)
3.2 Which compound (A, B or C) will have the highest vapour pressure at a given temperature? Refer to the data in the table to explain the answer. (2)
3.3 For this investigation, write down the:
3.3.1 Independent variable (1)
3.3.2 Controlled variable (1)
3.4 Explain the trend in boiling point in the table above by referring to the MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY involved. (4)
3.5 The compound, 2-methylpropan-1-ol is a CHAIN ISOMER of one of the compounds in table.
3.5.1 Write down the structural formula for 2-methylpropan-1-ol. (2)
3.5.2 Which compound (A, B, or C) in the table above is a CHAIN ISOMER of 2-methylpropan-1-ol? Give a reason for the answer. (2)
3.5.3 How will the boiling point of 2-methylpropan-1-ol compare to the isomer named in QUESTION 3.5.2? Write only HIGHER THAN, LOWER THAN or EQUAL TO. (1)
3.5.4 Explain the answer to QUESTION 3.5.3 by referring to the MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY involved. (3)
3.6 Methanoic acid is a smaller compound than propan-1-ol. The boiling point of methanoic acid is HIGHER than propan-1-ol. Fully explain why the boiling point of methanoic acid is higher than propan-1-ol. Refer to the TYPE OF INTERMOLECULAR FORCES and energy involved. (4)
[22]
QUESTION 4 (Start on a new page.)
Hexane undergoes thermal cracking according to the balanced equation below.
C6H14 → Alkane X + C2H4
4.1 Define cracking. (2)
4.2 Write down the:
4.2.1 Molecular formula of alkane X (2)
4.2.2 One reaction condition (1)
The alkane X, produced in the cracking reaction above, is used to produce other organic compounds as shown in the flow diagram below.
The numbers I, II and III represent organic reactions.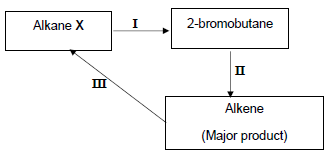
4.3 Write down the TYPE of reaction represented by:
4.3.1 I (1)
4.3.2 II (1)
4.4 Write down the NAME of the type of addition reaction represented by reaction III. (1)
4.5 Write down the NAME or FORMULA of the:
4.5.1 Inorganic reactant used in reaction I (1)
4.5.2 Catalyst used in reaction III (1)
4.6 Write down a balanced equation using CONDENSED structural formulae of organic reagents for reaction II. (5)
[15]
QUESTION 5 (Start on a new page.)
A group of learners conduct experiments to investigate a factor that affects the rate of reactions.
In the experiments, the learners use the reaction between zinc granules and EXCESS hydrochloric acid of concentration 0,5 mol∙dm-3 at 20 °C as shown below:
Experiment A: Zn (s) + 2 HCℓ (aq) → ZnCℓ2 (aq) + H2 (g) ΔH < 0
5.1 Define reaction rate. (2)
5.2 Is net energy ABSORBED or RELEASED during the reaction? Give a reason for the answer. (2)
5.3 How will the rate of reaction in experiment A be affected by the following changes? Choose from INCREASES, DECREASES or NO EFFECT.
5.3.1 Using H2SO4 solution of concentration 0,5 mol∙dm-3 in place of the HCℓ. (1)
5.3.2 Pressure is increased. (1)
5.4 In experiment A, 50 cm3 of hydrochloric acid (HCℓ) solution of concentration 0,5 mol∙dm-3 reacts with 7,15 g of zinc (Zn) granules.
The volume gas formed versus time for the reaction in experiment A is shown below.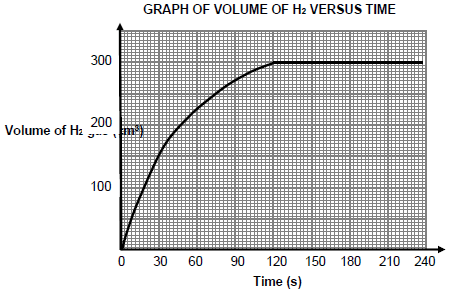
5.4.1 How many seconds did the reaction take to reach completion? (1) It is observed that the rate of reaction is HIGHEST during the interval 0 to 30 s.
5.4.2 Write down TWO factors that influence reaction rate that explains this observation. (2)
5.5 Calculate the:
5.5.1 Average rate of reaction (in cm3∙s-1) for the first 120 s (3)
5.5.2 Mass of the EXCESS reactant remaining in the flask if the molar volume of hydrogen gas at 20 °C is 24 dm3∙mol-1 (7)
5.6 In experiment B, the reaction in experiment A is repeated under the same conditions, but copper is also added to the reaction mixture.
Cu
Experiment B: Zn (s) + HCℓ (aq) → ZnCℓ2 (aq) + H2 (g)
The Maxwell-Boltzman distribution curve for the reaction in experiment A and in experiment B is shown below: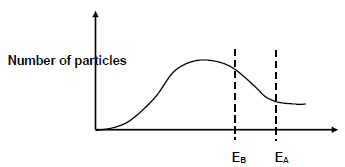
EA and EB represent the activation energy for the reaction in experiments A and B respectively.
5.6.1 What is the function of copper in experiment B? (1)
5.6.2 Explain how the addition of copper in reaction B affects the rate of reaction by referring to the collision theory. (4)
[24]
QUESTION 6 (Start on a new page.)
The reversible reaction given below reaches equilibrium at 350 °C in a closed container.
N2(g) + 3 H2(g) ⇋ 2 NH3(g) ΔH < 0
6.1 Define the term reversible reaction. (2)
6.2 The temperature of the equilibrium mixture is decreased. How will the decrease in temperature affect the following? Choose from INCREASES, DECREASES or REMAINS CONSTANT.
6.2.1 Rate of the forward reaction (1)
6.2.2 Number of moles of NH3 at equilibrium (1)
6.3 Explain the answer to QUESTION 6.2.2 above by referring to Le Chatelier’s principle. (2)
6.4 The reaction is started by placing 7,84 grams of N2 and 0,6 moles of H2 in an empty flask which is then sealed. When equilibrium is reached at 350 °C the amount of NH3 present is 0,12 moles. The volume of the container is 2 dm3. Calculate the value of the equilibrium constant at 350 °C. (8)
The graph shown below shows the percentage yield of NH3 at 350 °C at different pressure values.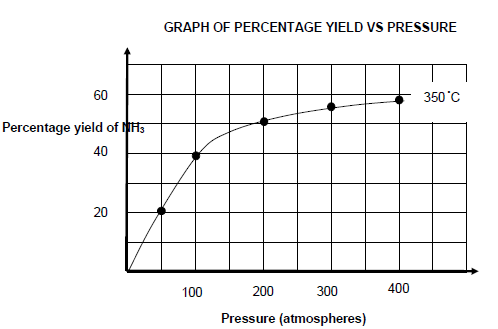
6.5 Write down a CONCLUSION that can be drawn from the graph about the relationship between percentage yield of NH3 and pressure at constant temperature. (2)
6.6 Use information from QUESTION 6.4 and the graph to determine the pressure at which the reaction reached equilibrium. (5)
[21]
QUESTION 7 (Start on a new page.)
7.1 Sodium carbonate ionises in water into sodium ions (Na+) and carbonate ions (CO32-). Carbonate ions in solution undergo hydrolysis according to the balanced equation:
CO32- (aq) + H2O (ℓ) ⇋ HCO3- (aq) + OH- (aq)
7.1.1 Define hydrolysis. (2)
7.1.2 Give a reason why a solution of sodium carbonate in water is alkaline by referring to substance(s) in the equation above. (2)
7.1.3 Write down the FORMULAE of the TWO acids in the equation. (2)
7.1.4 Give a reason why HCO3- can act as an ampholyte. (2)
7.2 A solution of a strong diprotic acid X has pH = 1.
7.2.1 Define an acid according to the Lowry-Bronsted theory. (2)
7.2.2 Calculate the concentration of acid X. (4) A solution of acid X of concentration 0,049 mol.dm-3 is diluted by adding 15 cm3 of the acid to water to produce 90 cm3 of a dilute standard solution.
7.2.3 Explain the meaning of the term standard solution. (2)
The DILUTE solution of acid X is titrated with a solution of potassium hydroxide.
The following list of indicators are available for the titration.
Indicator | pH-range |
Methyl orange | 3,1 – 4,4 |
Bromothymol blue | 6,0 – 7,6 |
Phenolphthalein | 8,3 – 10,0 |
7.2.4 Which ONE of the indicators is the most suitable for this titration? Explain the answer. (3)
During the titration 25 cm3 of the DILUTE acid X solution neutralises exactly 28,5 cm3 of a potassium hydroxide solution.
7.2.5 Calculate the concentration of the potassium hydroxide solution. (7)
[26]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
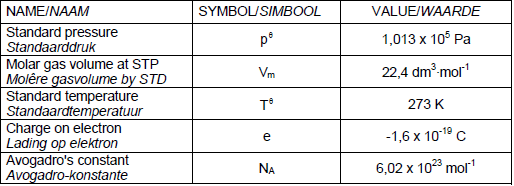
TABLE 2: FORMULAE 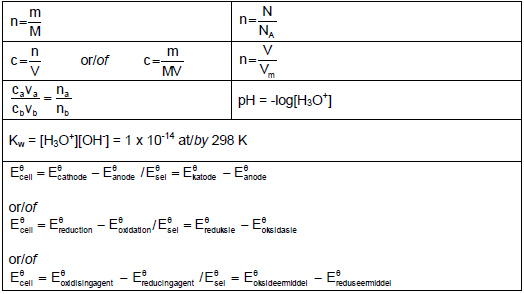
PERIODIC TABLE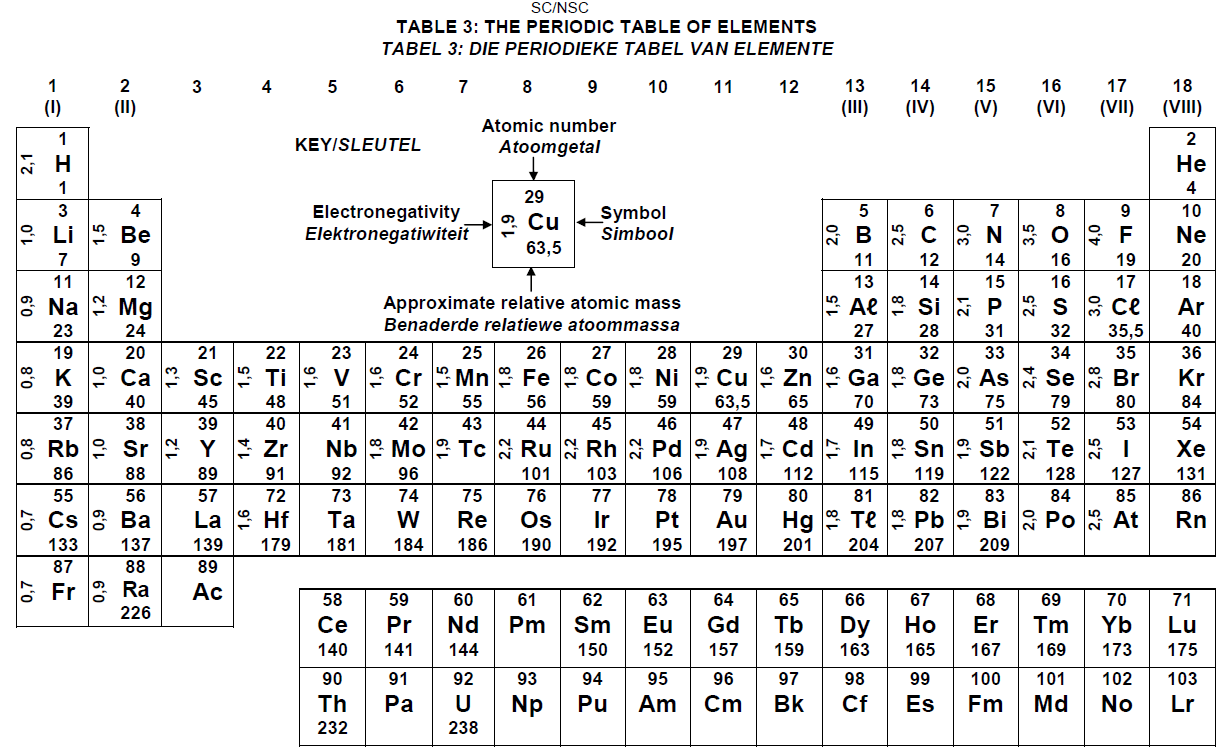
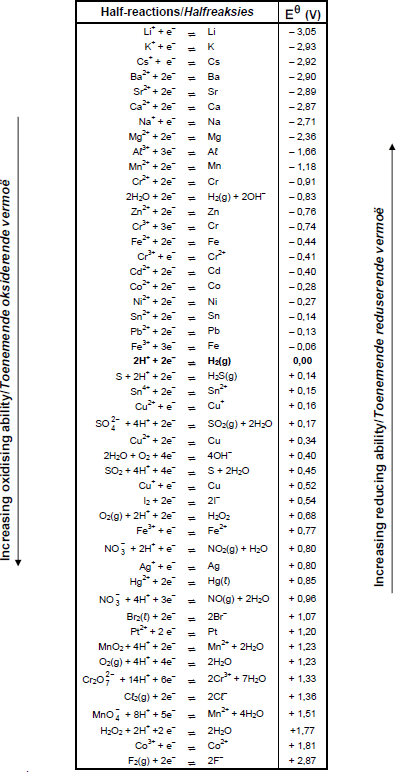
TABLE 4A: STANDARD REDUCTION POTENTIALS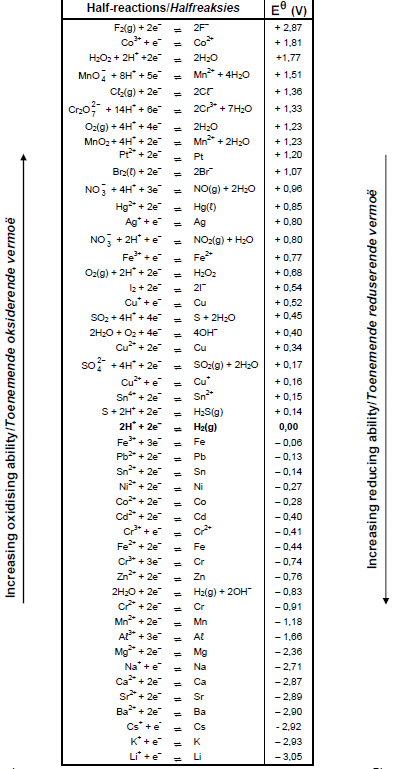
TABLE 4B: STANDARD REDUCTION POTENTIALS