TECHNICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC EXAMS PAST PAPERS AND MEMOS NOVEMBER 2020
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NOVEMBER 2020
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
QUESTION 1
1.1 B ✓✓(2)
1.2 C ✓✓ (2)
1.3 D ✓✓ (2)
1.4 A ✓✓ (2)
1.5 A ✓✓ (2)
1.6 D ✓✓(2)
1.7 B ✓✓ (2)
1.8 C ✓✓ (2)
1.9 C ✓✓ (2)
1.10 D ✓✓ (2)
[20]
QUESTION 2
2.1 An atom or a group of atoms (bond) that determine the chemistry of a molecule.✓✓
OR
An atom or a group of atoms (bond) that determine(s) the physical and chemical properties of a group of organic compounds. (2)
2.2.1 Alkenes (1)
2.2.2 Aldehydes (1)
2.2.3 Carboxylic acids (1)
2.3.1 Hexanal (2)
2.3.2 Propanoic acid (2)
2.4.1 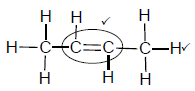
Marking criteria:
- Double bond ✓
- Whole structure correct. ✓
Note: Any hydrogen atom or bond missing or added Max: 1/2
(2)
2.4.2 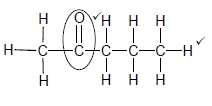
Marking criteria:
- Functional group ✓
- Whole structure correct✓
Note: Any hydrogen atom or bond missing or added Max 1/2
2.5.1 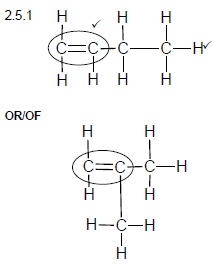
Marking criteria:
- Double bond ✓
- Whole structure correct.✓
Note: Any hydrogen atom or bond missing or too many bonds Max: 1/2 (2)
2.5.2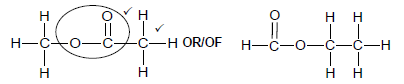
Marking criteria:
- Correct functional group ✓
- Whole structure correct. ✓
Note: Any hydrogen atom or bond missing or added Max ½
2.6.1 Negative marking from 2.5.1
Positional ✓ (if but-1-ene is given in 2.5.1).
OR
Chain/positional isomer (if 2-methyl prop-1-ene is given in 2.5.1) (1)
2.6.2 Functional (1)
2.7.1 Unsaturated (1)
2.7.2 Negative marking from 2.7.1
It contains carbon - carbon double/multiple bond (1)
[21]
QUESTION 3
3.1 The pressure exerted by a gas in equilibrium with a (solid or) liquid in a closed container/closed system (at a given temperature). (2)
3.2.1
- Vapour pressure decreases with an increase in molar mass/molecular mass/number of C-atoms/chain length.✓
OR - Vapour pressure increases with a decrease in molar mass/molecular mass/number of C-atoms/chain length.
OR - Vapour pressure decreases from 1-propanol to 1-pentanol.
OR - Vapour pressure increases from 1-pentanol to 1-propanol. (1)
3.2.2
- Chain length/molar mass increases from 1-propanol to 1-pentanol. ✓
- The strength of intermolecular forces increases from 1-propanol to 1-pentanol/with increase in chain length/molar mass✓
- More energy is needed to overcome strong intermolecular forces from 1-pentanol to 1-propanol. ✓
OR - Chain length/molar mass decreases from 1-pentanol to 1-propanol.
- The strength of intermolecular forces decreases from 1-pentanol to 1-propanol/with decrease in chain length/molar mass area.
- Less energy is needed to overcome weak intermolecular forces from 1-propanol to 1-pentanol. (3)
3.3.1
- Aldehydes /A:
- Alkanes /B: (2)
3.3.2
- A/Aldehydes have (London forces) and dipole-dipole forces. B/Alkanes contain London forces only. ✓
- Dipole-dipole forces/forces in A/aldehydes are stronger than London forces/forces in B/ alkanes. ✓
OR - London forces/Forces in B/alkanes are weaker than dipole-dipole forces /forces in A/aldehydes.
OR - The intermolecular forces in A are stronger than the intermolecular forces in B.
OR - The intermolecular forces in B are weaker than the intermolecular forces in A.
- Aldehydes need more energy to overcome the intermolecular forces and therefore have higher boiling points. ✓
OR - Aldehydes have higher boiling points than alkanes.
OR - Alkanes have lower boiling points than aldehydes. (3)
Marking criteria:
A/B or forces is only accepted if 3.3.1 is correctly identified.
[11]
QUESTION 4
4.1.1 Addition/hydration ✓ (1)
4.1.2 Addition/hydrohalogenation/hydrobromination✓(1)
4.1.3 Substitution/Halogenation (1)
4.2.1 Water/H2O ✓ (1)
4.2.2 (Dilute) sulphuric acid/H2SO4 OR (Dilute) phosphoric acid/H3PO4 (1)
4.3 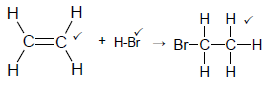
Note: Do not penalise HBr
No arrow max 2/3 (3)
4.4 Moderate/warm temperature ✓/Mild heat. (Accept: UV light).
No water✓ (Accept: concentrated H2SO4 + NaBr/HBr). (2)
4.5 C2H4 +3O2 → 2CO2 + 2H2O (+ energy)✓(balancing) (3)
Marking criteria:
- Reactants ✓
- Products ✓
- Balancing ✓
Note: Do not penalise if energy is omitted.
4.6 Ethene(1)
Note: Penalise if molecular formula is written
[14]
QUESTION 5
5.1 (A process) in which electrical energy is converted into chemical energy ✓✓
OR
The decomposition of a (ionic) substance when an electric current is passed through it. (2)
5.2 Non spontaneous (1)
5.3 Apply negative marking from 5.2.
It requires electrical energy✓
OR
It is an endothermic reaction (1)
5.4 CuCℓ2 (1)
Note: Penalise if name is written.
5.5.1 Anode (1)
5.5.2 Cathode (1)
5.6 Reducing agent – a substance that is oxidised/loses electrons. ✓✓
OR
Reducing agent – A substance that undergoes oxidation. (2)
5.7.1 2Cℓ- → Cℓ2 + 2e- (2)
Note: Penalise once if charge if left out on chloride ion
Marking criteria:
2Cℓ- → Cℓ2 + 2e- 2/2
2Cℓ- ⇌ Cℓ2 + 2e- 1/2
2Cℓ- ← Cℓ2 + 2e- 0/2
Cℓ2 + 2e- ⇌ 2Cℓ-- 0/2
5.7.2 Cu2+ + 2e- → Cu (2)
Note: Penalise once if charge if left out on copper ion
Marking criteria:
Cu2+ + 2e- → Cu 2/2
Cu2+ + 2e- ⇌ Cu 1/2
Cu ⇌ Cu2+ + 2e- 0/2
Cu2+ + 2e ← Cu 0/2
5.7.3 Cu2+ + 2Cℓ- → Cℓ2 + Cu (2)
Accept: CuCℓ2(aq) → Cℓ2 + Cu
[15]
QUESTION 6
6.1.1 An (electrochemical cell) that converts chemical energy to electrical energy. (2)
6.1.2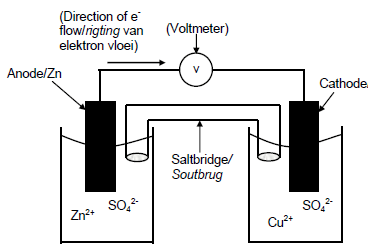
| CRITERIA FOR MARKING | |
| Anode in correct half cell, labelled (Zn electrode in Zn2+ /zinc sulphate or zinc nitrate solution) | ✓ |
| Cathode in correct half cell, labelled (Cu electrode in Cu2+ /copper sulphate or copper nitrate solution) | ✓ |
| Voltmeter included and labelled Accept: galvanometer | ✓ |
| Salt bridge included and labeled | ✓ |
| Correct direction of the flow electrons in the external circuit Note: if not labelled, do not penalise | ✓ |
(5)
Note: Credit the saltbridge and voltmeter only if separate containers.
6.1.3 Temperature/Temperatuur: 25°C/298 K ✓
Concentration of electrolytes : 1 mol∙dm-3 ✓(2)
6.1.4 Towards the zinc (half cell)/anode.
Accept: Zn/Zn2+(aq) (1)
6.1.5 To maintain electrical neutrality, ✓ because the Zinc half-cell becomes more positive as zinc is oxidised to form zinc ions/Zn2+.(2)
6.2.1
| OPTION 1 | OPTION 2 |
| Eθcell = Eθcathode - Eθanode 2,00= 0,34 - EθX EθX = - 1,66 V Electrode X is aluminium/Aℓ | X → Xn+ + ne- (y) Cu2+ + 2e- → Cu -(0,34) X + Cu2+ → Xn+ + Cu 2,00V EθX = - 1,66 V Electrode X is aluminium/Aℓ |
(5)
Note: Positive marking of answer obtained in 6.2.1 for 6.2.2, 6.2.3. and 6.2.4.
6.2.2 Aℓ → Aℓ3+ + 3e- (2)
Marking criteria
Aℓ → Aℓ3+ + 3e- 2/2
Aℓ ⇌ Aℓ3+ + 3e- 1/2
Aℓ3+ + 3e- ⇌ Aℓ 0/2
Aℓ ← Aℓ3+ + 3e- 0/2
6.2.3 X/Aluminium/ Aℓ/ anode/negative electrode ✓ (1)
6.2.4 X/Aluminium/Aℓ will be oxidised. ✓✓
OR
X/Aluminium/Aℓ will lose electrons. (2)
[22]
QUESTION 7
7.1.1 The change in direction✓ of a wave upon striking the interface ✓ between two materials.
OR
The change in direction of a wave front at the interface (boundary) between two media, bouncing back into the original medium. (2)
7.1.2 Incident (ray) (1)
7.1.3 Reflected (ray) (1)
7.1.4 Normal (1)
7.1.5 (Angle) of incidence (1)
7.1.6 (Angle) of reflection (1)
7.2 30° (1)
7.3 The speed will decrease. (1)
7.4.1 Water (1)
7.4.2 It will bent towards the normal/It will be refracted towards the normal
Accept: change direction (1)
7.4.3 Refraction (1)
7.5.1 (Critical angle) is the angle of incidence in the denser medium ✓ such that the refracted ray just passes through the surface of separation of the two media. ✓
OR
An angle of incidence in the denser medium whose angle of refraction is 90º (a right angle). (2)
7.5.2 Light ray will travel from water and pass into the air. ✓ It will be refracted away/move away from the normal✓ (2)
7.5.3 When the angle of incidence is greater than the critical angle ✓ the ray of light reflects (back) into the original medium (2)
7.5.4 Greater than (1)
[19]
QUESTION 8
8.1 (The phenomenon) whereby white light breaks up (spreads out) ✓ into its component colours. (2)
8.2 The different colours of visible light include:
- Red
- Orange
- Yellow
- Green
- Blue
- Indigo
- Violet
(Any FOUR) (4)
8.3.
- It is always virtual.
- Erect/upright.
- Its size is equal to that of the object.
- It is formed at the same distance behind the mirror as the object is in front of the mirror.
- It is laterally inverted.
(Any THREE) (3)
8.4.1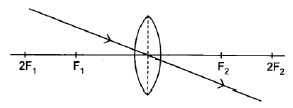
Note:
Penalise once if arrow is missing in answer 8.4.1, 8.4.2 and/or 8.4.3. (1)
Marking criteria:
Ray through the optical centre of the lens continues straight through the lens without deviation.
8.4.2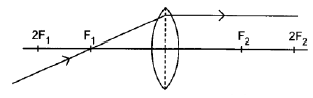
Marking criteria:
- Ray parallel to the principal axis on the opposite side of the lens without any refraction ✓
- Correct direction of ray (2)
8.4.3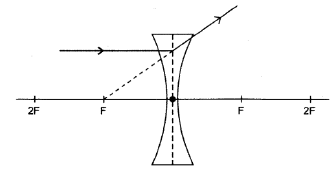
Marking criteria:
- Ray refracted and diverges on the opposite side of the lens ✓
- Correct direction of ray ✓
Note: Don’t penalise if dotted extrapolated line is omitted or is incorrectly drawn, but angle of refracted ray must be correct (2)
[14]
QUESTION 9
9.1 (Electromagnetic wave) is the changing of the magnetic and electric fields mutually perpendicular to each other and to the direction of propagation of the wave. (2)
9.2 They have longer wavelengths (1)
9.3 Quantum (packets) of energy (1)
9.4.1 Ultraviolet ✓ (1)
9.4.2 Infrared (1)
Accept: radio waves/
9.4.3 Radio waves
Accept: micro waves (1)
9.5 Wavelength and frequency are inversely proportional. ✓✓
OR
When wavelength becomes longer, frequency decreases.
OR
When wavelength becomes shorter, frequency increases (2)
9.6 OPTION 1
c = ƒ λ
3,0 x 108 = ƒ (4,06 x 10-11)
ƒ = 7,39 x 1018 Hz
E = hƒ
= (6,63 x 10-34)(7,39 x 1018)
= 4,90 × 10-15 J
Accept : 4,8990 × 10-15 J
OPTION 2
E = hc/λ
E = (6,63 x 10-34 )(3,0 x108)
4,06 x 10-11
E = 4,90 x10-15 J (5)
[14]
TOTAL: 150