PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC EXAMS PAST PAPERS AND MEMOS NOVEMBER 2020
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (PAPER 2)
GRADE 12
NATIONAL SENIOR CERTIFICATE
MEMORANDUM
NOVEMBER 2020
QUESTION 1
1.1 C ✓✓(2)
1.2 D ✓✓ (2)
1.3 C ✓✓ (2)
1.4 B ✓✓ (2)
1.5 D ✓✓(2)
1.6 B ✓✓ (2)
1.7 B ✓✓ (2)
1.8 C ✓✓(2)
1.9 A✓✓ (2)
1.10 C ✓✓ (2)
[20]
QUESTION 2
2.1.1 Ketones✓(1)
2.1.2 Pentanal✓✓
ACCEPT
2,2-dimethylpropanal
2-methylbutanal
3-methylbutanal
Marking criteria
- Correct functional group,i.e. –al
- Whole name correct (2)
2.2.1 5 – bromo-2,3 – dimethylhexane
Marking criteria:
- Correct stem i.e. hexane
- All substituents (bromo and dimethyl) correctly identified.
- IUPAC name completely correct including numbering, sequence, hyphens and commas. (3)
2.2.2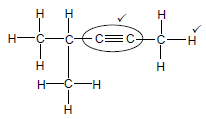
Marking criteria
- Whole structure correct 2/2
- Only functional group correct: ½
IF
More than one functional group 0/2 (2)
IF
Molecular formula 0/2
Condensed structural formula ½
2.3.1 The C atom bonded to the hydroxyl group is bonded to only one other C-atom. ✓✓ (2 or 0)
OR
The hydroxyl group/-OH/ is bonded to a C atom which is bonded to two hydrogens atoms. (2 or 0)
OR
The hydroxyl group/functional group/-OH is bonded to:
a primary C atom / the first C atom (2 or 0)
OR(2)
2.3.2 Esterification/condensation ✓(1)
2.3.3 Butanoic acid ✓(1)
[12]
QUESTION 3
3.1 Marking criteria
If any one of the underlined key phrases in the correct context is omitted, deduct 1 mark.
The temperature at which the vapour pressure equals atmospheric (external) pressure. ✓✓ (2)
3.2 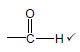 (1)
(1)
3.3
- Increase in the number of C-atoms increases molecular mass/size/chain length/surface area. ✓
- Strength of the intermolecular forces increases/More sites for London forces. ✓
- More energy is needed to overcome/break intermolecular forces. ✓ (3)
3.4.1 C ✓ (1)
3.4.2 B ✓
Marking criteria
- Compare strength of intermolecular forces of A, B and C. ✓✓
- Compare boiling points/energy required to overcome intermolecular forces of alcohols/A and aldehydes/B. ✓
OR
Alcohols have the highest boiling point. - Compare boiling points/ energy required to overcome intermolecular force of aldehydes/B and alkanes/C .✓
OR
Alkanes have the lowest boiling point.
Aldehydes/B have (in addition to London forces) dipole-dipole forces which are stronger than London forces, but weaker than hydrogen bonds. ✓
Therefore aldehydes/B have lower boiling points/require less energy to overcome intermolecular forces than alcohols/A,✓but higher boiling points / require more energy to overcome intermolecular forces than alkanes/C. ✓
OR
Aldehydes/B have stronger intermolecular forces than alkanes, but weaker intermolecular forces than alcohols/A. ✓
Therefore aldehydes/B have higher boiling points/ more energy required to overcome intermolecular forces than alkanes/C, ✓ but lower boiling points/ less energy to overcome intermolecular forces than alcohols/A.✓ (4)
3.5 Butanal ✓✓
Marking criteria
- Correct stem, i.e. but ✓
- Whole name correct(2)
3.6 Pentan-1-ol ✓✓
OR
1-pentanol ✓✓(2)
[15]
QUESTION 4
4.1 Marking criteria
- Addition reaction / reaction of alkene / reaction of C – C double bond /reaction of unsaturated hydrocarbon✓
- (Addition of) hydrogen halide/HX/ hydrogen and halide. ✓
The addition of a hydrogen halide/HX✓to an alkene. (2)
4.2 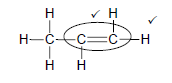
Marking criteria
- Whole structure correct 2/2
- Only functional group correct: ½
(2)
4.3.1 Cracking ✓ (1)
4.3.2 C8H18 ✓ (1)
4.4 1,2–dibromo ✓ propane ✓
1,2-dibromopropaan (2)
4.5.1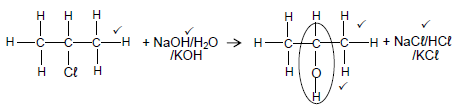
Marking criteria for the alcohol
- Whole structure correct 2/2
- Only functional group correct: ½
Notes:
- If 1-chloropropane used as reactant, 2 marks for the primary alcohol.
- Condensed or semi-structural formula: Max. 4/5
- Molecular formula:2/5
- Any additional reactants or products: Max.4/5
- If arrow in completely correct equation omitted: Max.4/5
- The product NaCℓ/KCℓ/HCℓ must be marked in conjunction with reactant NaOH/KOH/H2O. (5)
4.5.2
- (Mild) heat
- Dilute strong base/NaOH/LiOH/KOH OR water/H2O ✓ (2)
[15]
QUESTION 5
5.1.1 (Reaction) rate(1)
5.1.2 Surface area/state of division /particle size (1)
5.2.1 (Decreasing gradient indicates) rate of reaction is decreasing. ✓(1)
5.2.2 (Gradient is zero, indicates) reaction rate is zero ✓(1)
5.3
ave rate =ΔV/Δt
= 500 (-0) = 8,33 (cm3∙s-1)
60 (-0)
(3)
5.4 Equal to (1)
5.5 Greater than
Experiment C:
- Surface area of CaCO3 powder is greater than that of CaCO3 granules./
More particles are exposed /More particles with correct orientation ✓ - More effective collisions per unit time/Higher frequency of effective collisions. ✓
- Increase in reaction rate.✓
OR
Experiment A:
- Surface area of CaCO3 granules is smaller/Fewer particles are exposed
(than that of powdered CaCO3). Less particles with correct orientation ✓ - Less effective collisions per unit time./Lower frequency of effective collisions. ✓
- Decrease in reaction rate.✓ (4)
5.6 Marking criteria:
- Divide volume by 25,7 in n = V/VM
If no substitution step shown, award mark for answer: 0,0195 mol - Ratio: n(CO2) = n(CaCO3).
- Substitute 100 in n = n/M or in ratio
- Final answer: 1,95 g to 2 g.
OPTION 1
n(CO2) = V/Vm = 0,5/25,7
= 0,0195 mol
n(CaCO3) = n(CO2) = 0,0195 mol ✓
m(CaCO3) = nM
= 0,0195(100)
= 1,95 g ✓
OPTION 2
25,7 dm3 .........1 mol
0,5 dm3 ..........0,0195 mol ✓
100 g ✓...........1 mol
x ....................0,0195 mol ✓
x = m(CaCO3) = 1,95 g ✓
OPTION 3
n(CO2) = V/Vm = 0,5/25,7
= 0,0195 mol
0,0195 mol CO2 ≡ 0,856 g CO2 ✓
m(CO2) produced : m(CaCO3)
44 g : 100 g
0,856 : x
x = 1,95 g CaCO3 (4)
[16]
QUESTION 6
6.1 Products can be converted back to reactants. ✓
OR
Both forward and reverse reactions can take place.
OR
A reaction which can take place in both directions. (1)
6.2.1 Remains the same(1)
6.2.2 Increases (1)
6.3
- (When pressure is increased) the reaction that leads to the smaller amount of gas / side with less molecules/number of moles is favoured.✓
- The reverse reaction is favoured. (2)
6.4 Endothermic
- Kc decreases with decrease in temperature. ✓
- Reverse reaction is favoured. / Concentration of reactants increases. / Concentration of products decreases./Yield decreases✓
- Decrease in temperature favours an exothermic reaction. ✓
OR - Kc increases with increase in temperature. ✓
- Forward reaction is favoured. / Concentration of reactants decreases. / Concentration of products increases./Yield increases ✓
- Increase in temperature favours an endothermic reaction.✓ (4)
6.5 CALCULATIONS USING NUMBER OF MOLES
Mark allocation
- Correct Kc expression (formulae in square brackets).✓
- Substitution of equilibrium concentrations into Kc expression. ✓
- Substitution of Kc value. ✓
- Multiply equilibrium concentrations of I2 and I by 12,3 dm3. ✓ (OPTION 1)
- Multiply equilibrium concentrations of I by 12,3 dm3 and divide equilibrium mol of I2 by 12,3 dm3. ✓(OPTION 2)
- Change in n(I) = n(I at equilibrium). ✓
- USING ratio: I2 : I = 1 : 2 ✓
- Initial n(I2) = equilibrium n(I2) + change in n(I2).✓
- Substitute 254 g·mol-1 as molar mass for I2.✓
- Final answer: (26 g - 27,94 g). ✓
OPTION 1
Kc = [I] 2
[I2]
3,76x10-3 =(4,79 x 10-3)2
[I2]
[I2] = 6,102 x 10-3 mol∙dm-3
| I2 | I | |
| Initial mass (g) | (0,1045)(254)✓ = 26,543 g ✓ | |
| Initial quantity (mol) | 0,1045 | 0 |
| Change (mol) | 0,0295 | 0,0589 |
| Quantity at equilibrium (mol) | 0,0751 | 0,0589 |
| Equilibrium concentration (mol∙dm-3) | 6,102 x 10-3 | 4,79 x 10-3 |
OPTION 2
| I2 | I | |
| Initial mass (g) | x | 0 |
| Change in amount (moles) | 0,0295 | 0,0589 |
| Equilibrium amount (moles) | x - 0,0295 | 0,0589 |
| Equilibrium concentration (mol∙dm-3) | x - 0,0295 12.3 | 4,79 x 10-3 (x 12.3 and divide by 12.3) |
Kc = [I] 2
[I2]
3,76x10-3 =(4,79 x 10-3)2
x - 0.0295
12.3
x = 0,1045 mol
m = nM
= (0,1045)(254)
= 26,543 g ✓
Wrong Kc expression 6/9
No Kc expression, correct substitution 8/9
CALCULATIONS USING CONCENTRATION
Mark allocation
- Correct Kc expression (formulae in square brackets). ✓
- Substitution of equilibrium concentrations into Kc expression. ✓
- Substitution of Kc value ✓
- Change in n(I) = n(I at equilibrium). ✓
- USING ratio: I2 : I = 1 : 2 ✓
- Initial [I2] = equilibrium [I2] + change in [I2]. ✓
- Divide by 12,3 dm3. ✓
- Substitute 254 g·mol-1 as molar mass for I2.✓
- Final answer 26,543 g. ✓
OPTION 3
Kc = [I] 2
[I2]
3,76x10-3 =(4,79 x 10-3)2
[I2]
[I2] = 6,102 x 10-3 mol∙dm-3
| I2 | I | |
| Initial mass (g) | 8,497x10-3 | 0 |
| Change in amount (moles) | 2,395x10-3 | 4,79x10-3 |
| Equilibrium concentration (mol∙dm-3) | 6,102 x 10-3 | 4,79 x 10-3 |
c = m
MV
8.497 x 10-3 = m
(254)(12.3)
m = 26.546g
(9)
[18]
QUESTION 7
7.1.1 Weak
Ionises/Dissociates incompletely/partially (in water) (2)
7.1.2 OPTION 1
pH = -log[H3O+]
3,85 = -log[H3O+]
[H3O+] = 1,41 x 10-4 mol∙dm-3
OPTION 2
[H3O+] = 10-pH
= 10-3,85
= 1,41 x 10-4 mol∙dm-3 (3)
7.1.3 Greater than (1)
7.1.4 CH3COO-(aq) + H2O(ℓ) ⇌ CH3COOH(aq) + OH-(aq)
OR
CH3COONa(aq) + H2O(ℓ) ⇌ CH3COOH(aq) + NaOH(aq)
Due to formation of hydroxide/OH- / the solution is basic/alkaline /pH > 7. (3)
7.2.1 Marking criteria
Substitute/vervang: 1 x 0,0145 OR 1 x 14,5 in c = n / ca x Va = na
V cb x Vb nb
- Use: n(CH3COOH) : n(NaOH) = 1:1
- Final answer/Finale antwoord: 0,0145 mol
OPTION 1
n(NaOH)reacted = cV
= 1(0,0145)
= 0,0145 mol
n(CH3COOH)diluted = n(NaOH)
= 0,0145mol (3)
7.2.2 POSITIVE MARKING FROM 7.2.1.
Marking criteria
- Calculate mass CH3COOH in 25 cm3 (1,13 g). ✓
- Formula: n = m/M
- Substitute: M = 60 g∙mol-1.
- n(CH3COOH)reacted = ninitial- nunreacted
- USE mol ratio: n(CaCO3) : n(CH3COOH) = 1 : 2.✓
- Substitution of 100 g∙mol-1 in m = nM. ✓
- Calculate percentage: 0,217 x 100
1,2 - Final answer: 18,08% (17,92 – 22,92)
m(CH3COOH) = 4,52 x 25 = 1,13 g
100
n(CH3COOH)ini. = m/M
=1,13= 0,01883 mol
60
n(CH3COOH)rea = 0,01883 – 0,0145 = 0,0043 mol
n(CaCO3) = ½n(CH3COOH)
= 0,5(0,0043)
= 0,00217 mol
m(CaCO3) = nM
= 0,00217(100) = 0,217 g
% CaCO3= 0,217 x 100
1,2
= 18,08 % (8)
[20]
QUESTION 8
8.1 Provides path for movement of ions./Ensures(electrical)neutrality in the cell. (1)
8.2 (The electrode) where oxidation takes place/electrons are lost.(2)
8.3 Mg/Magnesium (1)
8.4.1 2H++ 2e- → H2
Marking criteria
H2 ← 2H+ + 2e- (2/2) 2H+ + 2e- ⇌ H2 (½)
H2 ⇌ 2H+ + 2e- (0/2) 2H+ + 2e ← H2 (0/2)
- Ignore if charge omitted on electron.
- If charge (+) omitted on H+:
Example: 2H+ 2e- → H2 Max.:½ (2)
8.4.2 Magnesium/Mg ✓ (1)
8.5 OPTION 1
Eθcell = Eθreduction - Eθoxidation
= 0 - (- 2,36)
Eθcell = 2,36 V
Notes
- Accept any other correct formula from the data sheet.
- Any other formula using unconventional abbreviations, e.g.Eθcell= E°OA - E°RA followed by correct substitutions:
OPTION 2
2H++ 2e- → H2 Eθ = 0 V
Mg(s) → Mg2+(aq) + 2e- Eθ = +2,36 V
Mg(s) + 2H+(aq)→ Mg2+(aq) + H2(g) Eθ = +2,36 V (4)
8.6 H2 is a stronger reducing agent than Cu and therefore Cu2+/Cu ions are reduced/H2 is oxidised Electrons flow from H2 to Cu. (3)
[14 ]
QUESTION 9
9.1 ANY ONE:
- The chemical process in which electrical energy is converted to chemical energy. ✓✓ (2 or 0)
- The use of electrical energy to produce a chemical change. (2 or 0)
- Decomposition of an ionic compound by means of electrical energy. (2 or 0)
- The process during which and electric current passes through a solution/ionic liquid/molten ionic compound. (2 or 0) (2)
9.2 Battery/cell/ power source ✓(1)
9.3 Silver nitrate/AgNO3/ Silver ethanoate/CH3COOAg / Silver fluoride /AgF/ Silver perchlorate AgCℓO4. (1)
9.4 Remains the same
Rate of oxidation is equal to the rate of reduction. (2)
9.5 Ag → Ag+ + e-
Notes
Ag+ + e- ← Ag (2/2) Ag ⇌ Ag+ + e- (½)
Ag ← Ag+ + e- (0/2) Ag+ + e- ⇌ Ag (0/2)
- Ignore if charge omitted on electron.
- If charge (+) omitted on Ag+:
Example: Ag → Ag + e- ✓Max./Maks: (2)
[8]
QUESTION 10
10.1.1 (Liquid) Air (1)
10.1.2 Natural gas/methane/oil/coal/coke✓(1)
10.1.3 Iron/iron oxide/Fe/FeO (1)
10.1.4 NH3/Ammonia/Ammoniak ✓ (1)
10.1.5 Ostwald (process)/Ostwald(proses) ✓(1)
10.1.6 NH3 + HNO3 → NH4NO3 Bal
Marking criteria
- Reactants Products Balancing
- Ignore double arrows
- Marking rule 6.3.10.
10.2.1 NPK ratio/Ratio of primary nutrients ✓ (1)
10.2.2 OPTION 1
4/9 x X/100 x 20 = 2,315 kg
X = 26 (26,04)
OPTION 2
m(P) = 2,315 kg
Mass of 1 part P =2,315= 0,57575
4
Mass of N = (0,57575)(2) = 1,1575 kg
Mass of K = (0,57575)(3) = 1,73625 kg
Total mass of fertiliser:
1,1575 + 2,315 + 1,73625 = 5,20875 kg ✓
X =5,20875 x 100= 26,04 (3)
20
[12]
TOTAL: 150