Physical Sciences Paper 2 Questions - Grade 12 September 2021 Preparatory Exams
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 E.
1.1 Which ONE of the following is an ALKANE?
- C6H8
- C6H10
- C6H12
- C6H14 (2)
1.2 Esters are formed by a reaction between two organic compounds, X and Y, each with a different functional group. The functional groups of these compounds are: (2)
Compound X | Compound Y | |
A | Hydroxyl group | Carboxyl group |
B | Hydroxyl group | Carbonyl group |
C | Hydroxide ion | Carboxyl group |
D | Hydroxide ion | Carbonyl group |
1.3 When butane is subjected to high temperatures and pressures, the following reaction takes place:
Butane → methane + Y
Which ONE of the following represents Y?
- CHCCH3
- CH2CHCH3
- CH3CH2CH3
- CH3CHCHCH3 (2)
1.4 A hydrochloric acid solution, HCℓ(aq), of concentration 1 mol∙dm-3 is added to EXCESS POWDERED magnesium at 25 °C. Curve I below represents the volume of hydrogen gas produced during the reaction. Curve II was obtained at different conditions using the SAME VOLUME of hydrochloric acid solution.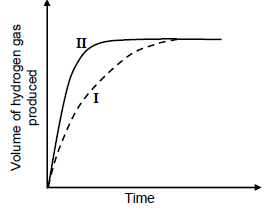
Which ONE of the following represents the conditions used to obtain curve II?
STATE OF DIVISION OF Mg | CONCENTRATION OF ACID | TEMPERATURE (°C) | |
A | Ribbon | 0,5 | 25 |
B | Ribbon | 2 | 25 |
C | Powder | 1 | 20 |
D | Powder | 1 | 30 |
(2)
1.5 In which ONE of the following reactions at equilibrium will the YIELD of the product increase when the VOLUME of the container is increased at constant temperature?
- N2O4(g) ⇌ 2NO2(g)
- H2(g) + I2(g) ⇌ 2HI(g)
- N2(g) + 3H2(g) ⇌ 2NH3(g)
- 2SO2(g) + O2(g) ⇌ 2SO3(g) (2)
1.6 Which ONE of the following statements is TRUE for an EXOTHERMIC reaction?
- More energy is absorbed than released.
- More energy is released than absorbed.
- Heat of reaction (ΔH) is positive.
- Energy of the products is greater than the energy of the reactants. (2)
1.7 Consider the equation below.
H3PO4(aq) + H2O(ℓ) ⇌ H3O+(aq) + H2PO-4 (aq)
Which ONE of the following is a conjugate acid-base pair?
- H3O+(aq) and H2O(ℓ)
- H3PO4(aq) and H2O(ℓ)
- H3PO4(aq) and H3O+(aq)
- H3O+(aq) and H2PO-4 (aq) (2)
1.8 Consider the balanced equation for the reaction below:
2Cr2+(aq) + Sn4+(aq) → 2Cr3+(aq) + Sn2+(aq)
The OXIDISING AGENT is:
- Cr2+(aq)
- Cr3+(aq)
- Sn2+(aq)
- Sn4+(aq) (2)
1.9 An electrochemical cell is set up at standard conditions. The cell notation for the cell is given below.
Mg(s) | Mg2+(aq) || Pb2+(aq) | Pb(s)
The cell is now connected in a circuit. Which ONE of the graphs below BEST represents the concentrations of the electrolytes after a long time?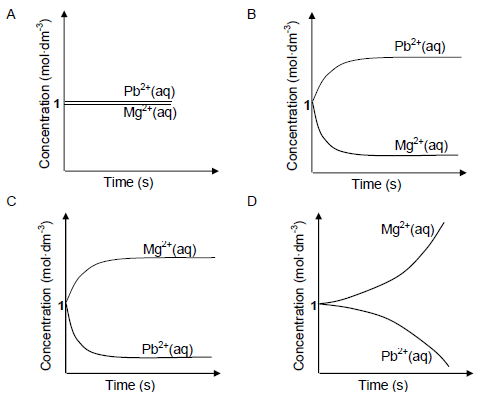 (2)
(2)
1.10 Two 50 kg bags, containing fertilisers R and S respectively, are labelled as follows:
Fertiliser R: 3 : 1 : 5 (20)
Fertiliser S: 1 : 2 : 6 (20)
Identify the fertiliser(s) most suitable for healthy leaf growth and healthy root growth.
LEAF GROWTH | ROOT GROWTH | |
A | R | R |
B | S | R |
C | R | S |
D | S | S |
(2) [20]
QUESTION 2 (Start on a new page.)
The letters A to E in the table below represent five organic compounds.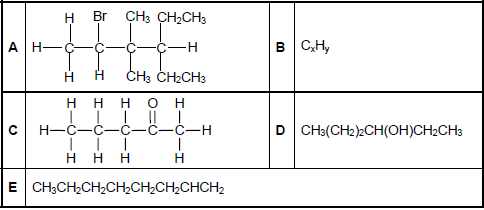
2.1 Write down the LETTER that represents EACH of the following:
2.1.1 A ketone (1)
2.1.2 A hydrocarbon (1)
2.1.3 An alkene (1)
2.2 Write down the:
2.2.1 IUPAC name of compound A (3)
2.2.2 STRUCTURAL FORMULA of compound D (2)
2.2.3 IUPAC name of the STRAIGHT CHAIN FUNCTIONAL ISOMER of compound C (2)
2.3 Compound B is a straight chain compound that undergoes the following exothermic reaction:
2CxHy + 25O2(g) → 16CO2(g) + 18H2O(g)
2.3.1 Besides being exothermic, what type of reaction is represented above? (1)
2.3.2 Determine the MOLECULAR FORMULA of compound B. (2)
The reaction above takes place in a closed container at a constant temperature higher than 100 °C and at constant pressure.
2.3.3 Calculate the TOTAL VOLUME of gas formed in the container when 50 cm3 of CxHy reacts completely with oxygen. (3)
[16]
QUESTION 3 (Start on a new page.)
Compounds A, B and C are used to investigate a factor which influences the boiling points of organic compounds. The results of the investigation are given in the table below.
COMPOUND | BOILING POINT (°C) | |
A | Butan-1-ol | 117 |
B | Butan-2-ol | 100 |
C | 2-methylpropan-2-ol | 82 |
3.1 Is this a fair investigation? Choose from YES or NO. (1)
3.2 Give a reason for the answer to QUESTION 3.1. (1)
3.3 Fully explain the difference in the boiling points of compounds B and C. (3)
3.4 Define the term positional isomer. (2)
3.5 From compounds A, B and C, choose the letter(s) that represent(s) EACH of the following:
3.5.1 Positional isomers (1)
3.5.2 A tertiary alcohol. Give a reason for the answer. (2)
3.6 The graph below represents the relationship between vapour pressure and temperature for compound A (butan-1-ol).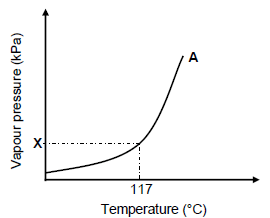
3.6.1 Write down the value of X. (1)
3.6.2 Redraw the graph above in the ANSWER BOOK. On the same set of axes, sketch the curve that will be obtained for compound C. Clearly label the curves A and C. Indicate the relevant boiling point for compound C on the graph. (2)
[13]
QUESTION 4 (Start on a new page.)
4.1 The flow diagram below shows various organic reactions using propane as starting reactant. R, T and U represent different organic compounds.
Compound T is a CARBOXYLIC ACID and compound U is a FUNCTIONAL ISOMER of pentanoic acid.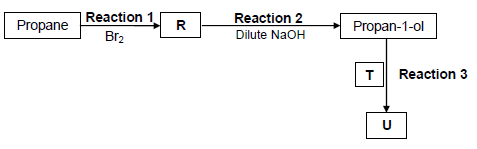
Write down the NAME of the type of reaction represented by:
4.1.1 Reaction 1 (1)
4.1.2 Reaction 2 (1)
Consider reaction 1 and reaction 2.
4.1.3 Write down the IUPAC name of compound R. (2)
Reaction 3 takes place in the presence of a catalyst and heat. Write down the:
4.1.4 NAME or FORMULA of the catalyst (1)
4.1.5 IUPAC name of compound T (2)
4.1.6 STRUCTURAL FORMULA of compound U (2)
4.2 A laboratory technician wants to prepare but-2-ene using but-1-ene as starting reagent, as shown below.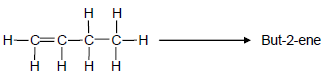
The following chemicals are available in the laboratory:
concentrated H2SO4 | H2O | concentrated NaOH |
Select the chemicals required to design this preparation from the list above.
For EACH step of the preparation, write down the balanced equation, using STRUCTURAL FORMULAE for all organic compounds. Indicate the chemicals needed in each step. (6)
[15]
QUESTION 5 (Start on a new page.)
5.1 Study the Maxwell-Boltzmann distribution curve for a certain reaction below.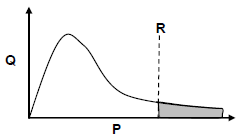
P and Q are the labels of the axes. What quantity is represented by:
5.1.1 P (1)
5.1.2 Q (1)
5.2 Line R represents the minimum energy required for the reaction to take place.
5.2.1 Write down the term for the underlined phrase. (1)
5.2.2 How will the shaded area on the graph be affected when a catalyst is added? Choose from INCREASE, DECREASE or REMAINS THE SAME. (1)
5.3 Use the collision theory to explain how a catalyst influences the rate of reaction. (4)
5.4 The reaction between POWDERED calcium carbonate, CaCO3(s), and EXCESS hydrochloric acid, HCℓ(aq), is used to investigate reaction rate at 25 °C. The balanced equation for the reaction is:
CaCO3(s) + 2HCℓ(aq) → CaCℓ2(aq) + H2O(ℓ) + CO2(g)
Several experiments are conducted using the same mass of IMPURE calcium carbonate and different initial concentrations of dilute hydrochloric acid. The graph below represents the results obtained. Assume that the impurities do not react.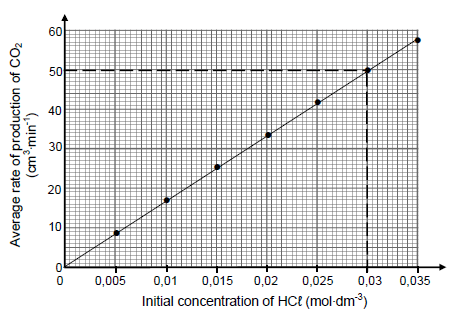
For this investigation, write down a:
5.4.1 Controlled variable (1)
5.4.2 Conclusion (2)
The CaCO3(s) in 6 g of the impure sample reacts completely with 0,03 mol∙dm-3 HCℓ(aq) in 26 minutes.
5.4.3 Use the information in the graph to calculate the percentage purity of the calcium carbonate. Assume that the molar gas volume at 25 °C is 24 000 cm3. (6)
[17]
QUESTION 6 (Start on a new page.)
Steam, H2O(g), reacts with hot carbon, C(s), at 1 000 °C according to the following balanced equation:
2H2O(g) + C(s) ⇌ 2H2(g) + CO2(g)
Initially, 36 g of steam and a certain amount of carbon were placed in a 2 dm3 sealed container and allowed to react. At equilibrium it was found that the amount of carbon changed by 0,225 mol.
6.1 Define the term dynamic equilibrium. (2)
6.2 Calculate the equilibrium constant, Kc, for the reaction at 1 000 °C. (8)
6.3 The graph shows how the rates of the forward and reverse reactions change with time.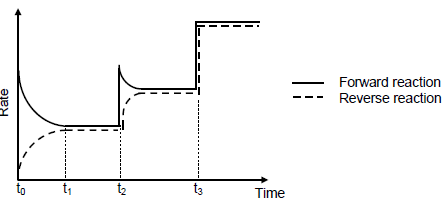
6.3.1 Give a reason why the rate of the forward reaction decreases between t0 and t1. (1)
6.3.2 What change was made to the equilibrium mixture at t3? (1) At time t2, the temperature of the system is increased.
6.3.3 Is the forward reaction EXOTHERMIC or ENDOTHERMIC? (1)
6.3.4 Refer to Le Chatelier’s principle to explain the answer to QUESTION 6.3.3. (2)
[15]
QUESTION 7 (Start on a new page.)
Two beakers, A and B, contain strong bases.
Beaker A: 500 cm3 of barium hydroxide, Ba(OH)2(aq) of unknown concentration X
Beaker B: 400 cm3 of potassium hydroxide, KOH(aq) of concentration 0,1 mol·dm-3
7.1 Define a base according to the Arrhenius theory. (2)
7.2 Calculate the number of moles of hydroxide ions ( OH-) in beaker B. (2)
7.3 The contents of beakers A and B are added together in beaker C. The solution in beaker C has a pH of 13. Assume that the volumes are additive and that the temperature of the solutions is 25 °C.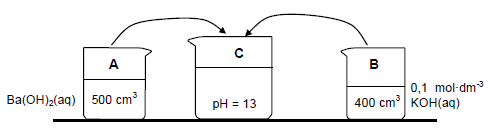
7.3.1 Calculate the concentration, X, of the Ba(OH)2 in beaker A. (8) The solution in beaker C is titrated with ethanoic acid. It was found that 15 cm3 of the solution neutralises 30 cm3 of the acid. The balanced equation for the reaction is:
CH3COOH(aq) + OH- (aq) → CH3COO- (aq) + H2O(ℓ)
7.3.2 Is ethanoic acid, CH3COOH(aq), a WEAK acid or a STRONG acid? Give a reason for the answer. (2)
7.3.3 Calculate the concentration of the ethanoic acid. (4)
[18]
QUESTION 8 (Start on a new page.)
A galvanic cell at standard conditions is represented by the cell notation below. X and Y are unknown electrodes.
X | Zn2+(aq) || Fe3+(aq) , Fe2+(aq) | Y
8.1 Write down the NAME or FORMULA of:
8.1.1 Electrode X (1)
8.1.2 Electrode Y (1)
8.1.3 The oxidising agent (1)
8.2 Write down:
8.2.1 ONE function of electrode Y (1)
8.2.2 The half-reaction that takes place at electrode Y (2)
8.2.3 The net (overall) equation for the cell reaction that takes place in this cell (3)
8.3 Calculate the initial emf of this cell. (4)
8.4 How will the initial emf of the cell be affected when the concentration of the iron(III) ions is changed to 0,6 mol∙dm-3? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
[14]
QUESTION 9 (Start on a new page.)
The simplified diagram below represents an electrochemical cell used for the purification of copper. The impure copper contains small amounts of silver (Ag) and zinc (Zn) as the only impurities.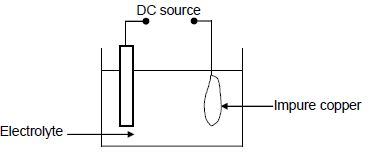
9.1 Define the term electrolysis. (2)
9.2 Write down the NAME or FORMULA of TWO positive ions present in the electrolyte. (2)
9.3 Write down the half-reaction that takes place at the cathode. (2)
9.4 Refer to the Table of Standard Reduction Potentials and explain why the purified copper will NOT contain any zinc. (3)
9.5 Calculate the maximum mass of Cu formed if 0,6 moles of electrons are transferred. (3)
[12]
QUESTION 10 (Start on a new page.)
10.1 The flow diagram below shows processes involved in the production of fertiliser C.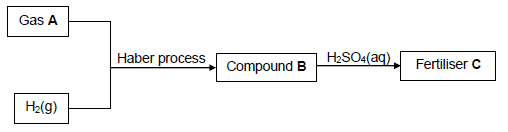
Write down the NAME or FORMULA of:
10.1.1 Gas A (1)
10.1.2 The catalyst used in the Haber process (1)
10.1.3 Compound B (1)
Write down the:
10.1.4 Name of the process used to produce gas A (1)
10.1.5 Balanced equation for the formation of fertiliser C (3)
10.2 A 40 kg bag of fertiliser contains 65% filler. The mass of the nutrients in the bag is shown in the table below.
NUTRIENTS | MASS (kg) |
Nitrogen | x |
Phosphorous | 2x |
Potassium | 5 |
Calculate the NPK ratio of the fertiliser. (3)
[10]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
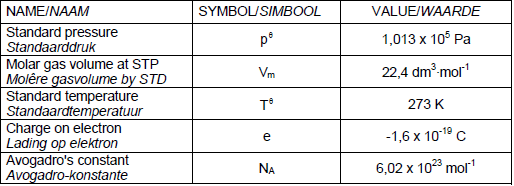
TABLE 2: FORMULAE 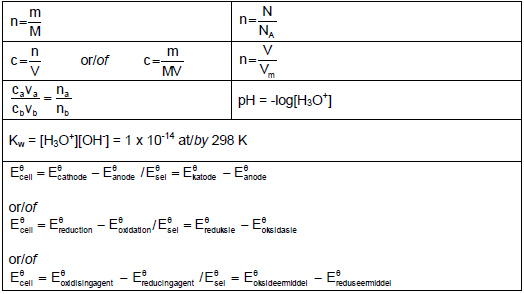
PERIODIC TABLE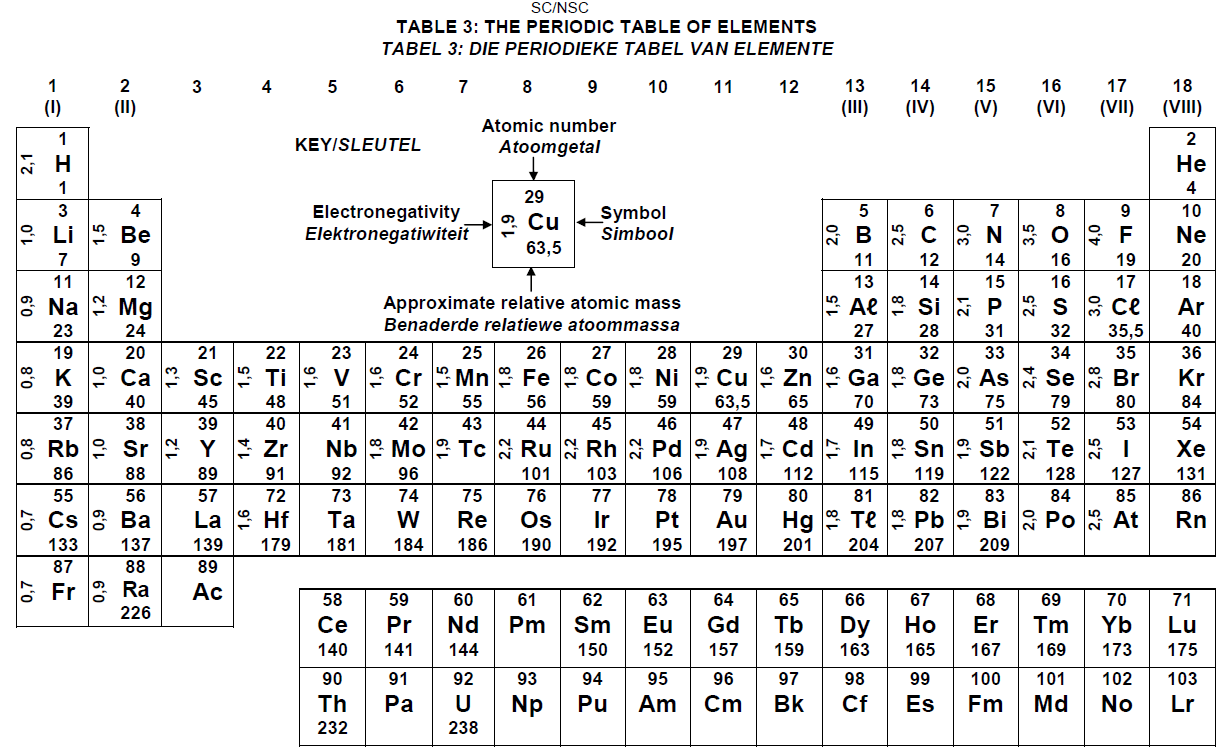
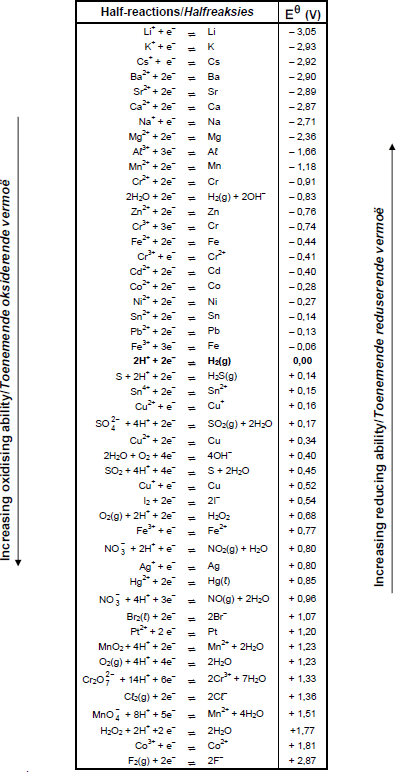
TABLE 4A: STANDARD REDUCTION POTENTIALS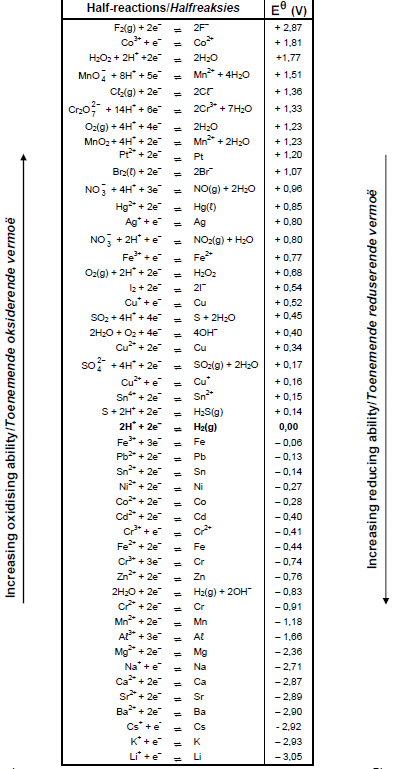
TABLE 4B: STANDARD REDUCTION POTENTIALS