TECHNICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC EXAMS PAST PAPERS AND MEMOS MAY/JUNE 2021
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE EXAMINATIONS
MAY/JUNE 2021
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You are advised to use the attached DATA SHEETS.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 D.
1.1 What is the IUPAC name of the organic molecule with the following structural formula: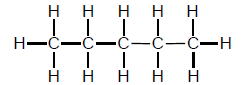
- Butane
- Methane
- Propane
- Pentane (2)
1.2 A compound has the following structural formula: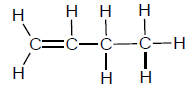
This compound is a/an …
- alkene.
- alcohol.
- haloalkane.
- carboxylic acid. (2)
1.3 The vapour pressure of ethanol at 25 °C in a closed container will increase when …
- the ethanol is cooled to 4 °C.
- the ethanol is heated to 86 °C.
- water is added to the ethanol at 25 °C.
- carboxylic acid is added to the ethanol. (2)
1.4 When butane burns in excess oxygen, the products formed are …
- methane and water.
- carbon and hydrogen.
- carbon dioxide and water.
- carbon monoxide and hydrogen. (2)
1.5 Consider the statements below about a galvanic cell.
- Reduction occurs at the cathode.
- The type of reaction taking place is a redox reaction.
- All electrochemical reactions involve the transfer of protons.
- All galvanic cells involve the use of electricity to initiate non-spontaneous chemical reactions.
Which ONE of the combinations below is INCORRECT?
- (iv) only
- (i) and (iii)
- (iii) and (iv)
- (ii), (iii) and (iv) (2)
1.6 In the standard cell notation for a voltaic cell, the single vertical line ' | ' represents a …
- phase boundary.
- wire connection.
- gas electrode.
- salt bridge. (2)
1.7 Molten NaCℓ conducts electricity due to the presence of …
- atoms of Na and Cℓ.
- free molecules.
- free electrons.
- free ions. (2)
1.8 Consider the diagram below. The letters a, b, c and d represent angles as indicated. Which ONE of the following is TRUE when light rays are reflected from a surface?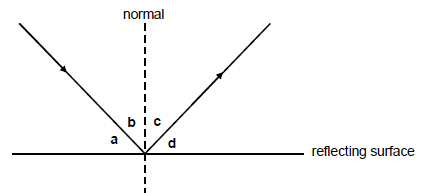
- ∠a = ∠c
- ∠b = ∠c
- ∠b = ∠d
- ∠a = ∠b (2)
1.9 The following devices use electromagnetic waves to function.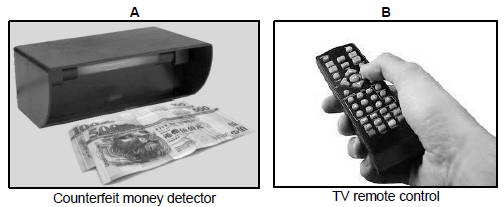
Which ONE of the combinations of properties of electromagnetic waves below is CORRECT?
| A | B |
| A. Long wavelength | Short wavelength |
| B. Low frequency | High frequency |
| C. Photons have high energy | Photons have low energy |
| D. Small amplitude | Large amplitude |
(2)
1.10 Which ONE of the following forms of energy production is NOT environmentally friendly?
- Hydroelectric energy
- Nuclear energy
- Solar energy
- Wind energy (2)
[20]
QUESTION 2 (Start on a new page.)
The questions below refer to the six organic compounds represented in TABLE 1 below.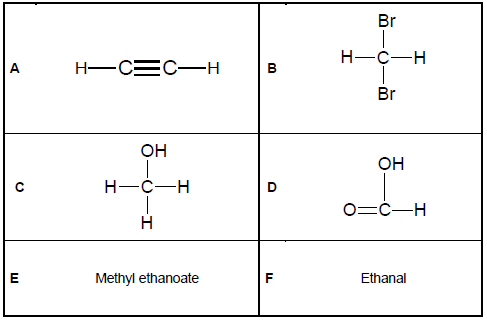
2.1 Define the term homologous series. (2)
2.2 Write down the LETTER of the compound belonging to the following homologous series:
2.2.1 Alcohols (1)
2.2.2 Carboxylic acids (1)
2.2.3 Alkynes (1)
2.2.4 Haloalkanes (1)
2.2.5 Esters (1)
2.3 Write down the NAME of the next member in the homologous series of compound F. (1)
2.4 Which of these compounds is used in the oxyacetylene flame during welding? (1)
2.5 Write down the structural formula of the FUNCTIONAL GROUP of the organic molecules represented by:
2.5.1 D (2)
2.5.2 F (2)
Consider the organic molecules in TABLE 2 below.
TABLE 2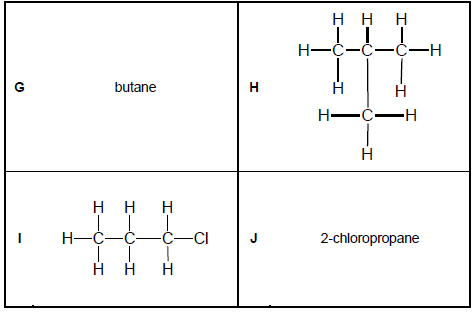
2.6 Differentiate between CHAIN and POSITIONAL isomers. (4)
2.7 Which of the organic molecules in TABLE 2 are:
2.7.1 Chain isomers (1)
2.7.2 Positional isomers (1)
2.8 Write down the IUPAC NAME of molecule:
2.8.1 H (2)
2.8.2 I (2)
2.9 Write down the structural formula of molecule J. (2)
[25]
QUESTION 3 (Start on a new page.)
3.1 Identify the type of intermolecular forces acting in:
3.1.1 Propane (1)
3.1.2 Propanal (2)
3.2 How does the strength of the intermolecular force influence the boiling point of organic molecules? (1)
3.3 3.3.1 Which one, ethanal or pentanal, will have the highest vapour pressure? (1)
3.3.2 Explain the answer to QUESTION 3.3.1. (4)
3.4 Below is the structural formula of a monomer.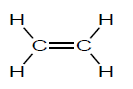
3.4.1 Define the term monomer. (2)
3.4.2 Write down the NAME of the polymer formed from the monomer given above. (1)
[12]
QUESTION 4 (Start on a new page.)
4.1 The equation below represents a reaction taking place in a sealed container. X represents an unknown reagent.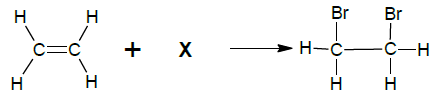
4.1.1 Write down the NAME or FORMULA of unknown reagent X. (1)
4.1.2 Write down the TYPE of reaction. Choose from ADDITION or SUBSTITUTION. (1)
4.1.3 Write down the NAME of the type of reaction in QUESTION 4.1.2. (1)
4.1.4 Write down the IUPAC name of the product formed. (2)
4.2 Refer to the following organic compounds:
TABLE 3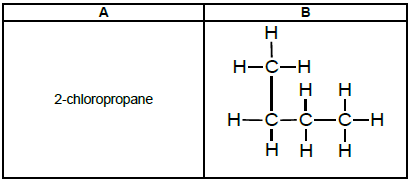
Which ONE of the compounds will be more likely to undergo an oxidation (combustion) reaction? Write down A or B. (1)
4.3 2-chloropropane undergoes a substitution reaction to form an alcohol.
4.3.1 Write down the TYPE of substitution reaction. (1)
4.3.2 Name ONE condition needed for this reaction. (1)
[8]
QUESTION 5 (Start on a new page.)
The diagram below represents an ELECTROLYTIC CELL used in the decomposition of copper (ll) chloride solution. Symbol X represents a component of the electrolytic cell. When the cell is in operation, cations (positive ions) are attracted to electrode A.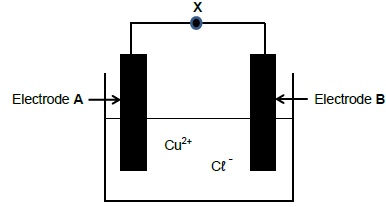
5.1 Write down the NAME of component X. (1)
5.2 Give a reason why component X is important in the cell above. (2)
5.3 Redraw the diagram above in your ANSWER BOOK and label both the anode and cathode, as well as component X. Indicate component X with the CORRECT symbol. (3)
5.4 Write down the chemical formula of the electrolyte. (1)
5.5 Compare the anode and the cathode with regard to the following:
5.5.1 Type of half-reaction(2)
5.5.2 Polarity of the electrode(2)
5.6 Write down the half-reaction that takes place at the negative electrode. (2)
5.7 Write down the net cell reaction for the cell above. (3)
[16]
QUESTION 6 (Start on a new page.)
A spontaneous electrochemical cell is set up under standard conditions using Ni and Ag as electrodes.
6.1 Choose ONE word from the statement above that indicates that the cell is a galvanic cell. (1)
6.2 Define the following:
6.2.1 Galvanic cell (2)
6.2.2 Electrolyte (2)
6.3 What energy conversion will take place in the cell above? (2)
6.4 Which ONE of the electrodes is the cathode? (1)
6.5 Write down the half-reaction at the cathode. (2)
6.6 Calculate the emf of the cell under standard conditions. (4)
6.7 Write down the cell notation for the cell above. (3)
6.8 Worldwide interest in renewable energy technologies continues to see strong growth each year. The outlook remains positive, especially for alternative solar energy technology applications.
6.8.1 Name ONE alternate energy source, besides solar energy. (1)
6.8.2 Name THREE uses of a photovoltaic cell. (3)
[21]
QUESTION 7 (Start on a new page.)
7.1 Mirrors produce images with a number of distinguishable characteristics. The diagram below shows an object and its image as observed in mirror MOM'.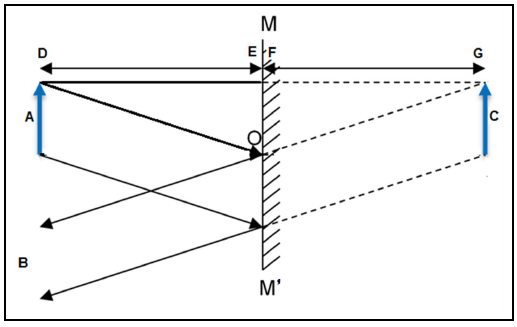
7.1.1 What type of a mirror is shown in the diagram above? (1)
7.1.2 Which phenomenon of light is demonstrated in the diagram? (1)
7.1.3 State the law of the phenomenon in QUESTION 7.1.2. (2)
7.1.4 Write down THREE properties of the image observed. (3)
7.2 Choose a word(s) from the list below that match(es) the letters A, B and C in the diagram above. Write only the word(s) next to the question numbers (7.2.1 to 7.2.3) in the ANSWER BOOK.
lens; object; mirror; normal; image; eye of the observer
7.2.1 A (1)
7.2.2 B (1)
7.2.3 C (1)
7.3 Consider the distances DE and FG in the diagram above.
7.3.1 What is the relationship between distances DE and FG? (1)
7.3.2 How will an increase in the size of object A influence distance FG? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
[12]
QUESTION 8 (Start on a new page.)
8.1 In the diagram below, a ray of light moves from water into air, with an angle of incidence of 49°.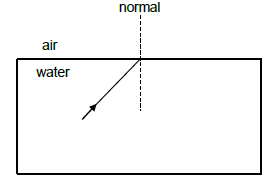
The critical angle for water/air is 49°.
8.1.1 What will be observed at the boundary of the two media when the incidence angle is equal to the critical angle?(1)
8.1.2 Redraw the diagram above in your ANSWER BOOK to indicate the path of the ray when the incident angle is increased to 65°. Label ALL rays and the magnitude of the angles. (3)
8.1.3 What is this phenomenon observed called? (1)
8.2 Dispersion is an observable property of light.
8.2.1 Define the term dispersion. (2)
8.2.2 Draw a labelled ray diagram of the dispersion of white light from air through a triangular prism. Indicate and name the colour that is refracted the MOST and the LEAST. (4)
8.2.3 How many OTHER colours are in the spectrum of white light, besides the two colours mentioned in QUESTION 8.2.2? (1)
8.2.4 Define the term refraction. (2)
8.2.5 Explain why white light spreads out into different colours when it passes through a triangular prism by referring to REFRACTION, WAVELENGTH and SPEED. (3)
[17]
QUESTION 9 (Start on a new page.)
9.1 Write down TWO differences between a concave lens and a convex lens. (4)
9.2 Some people's eyes are unable to focus on nearby objects, as indicated in the diagram below.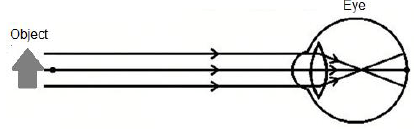
9.2.1 Write down the NAME of the eye condition as shown in the diagram above. (1)
9.2.2 Which type of lens can be used to correct the condition named in QUESTION 9.2.1? (1)
9.3 The diagram below shows the formation of an image when rays of light pass through an unknown lens. The object, the image, the optical centre and the principal axis are indicated.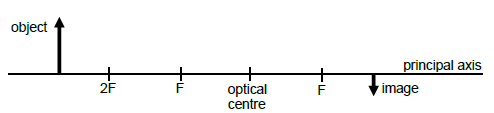
Redraw the diagram in your ANSWER BOOK. Indicate ALL the rays and the CORRECT type of lens. (5)
[11]
QUESTION 10 (Start on a new page.)
10.1 Define the term electromagnetic waves. (2)
10.2 The electromagnetic spectrum consists of the following electromagnetic waves: ultraviolet rays, microwaves, X-rays, gamma rays, radio waves, infrared rays.
Which electromagnetic wave has the:
10.2.1 Longest wavelength (1)
10.2.2 Highest frequency (1)
10.3 A photon of blue light has an energy of 1,75 x 10-48 J.
Determine, by means of a calculation, whether a photon of light with a frequency of 7,50 x 1014 Hz is a photon of GREEN light or of INDIGO light. (4)
[8]
TOTAL: 150
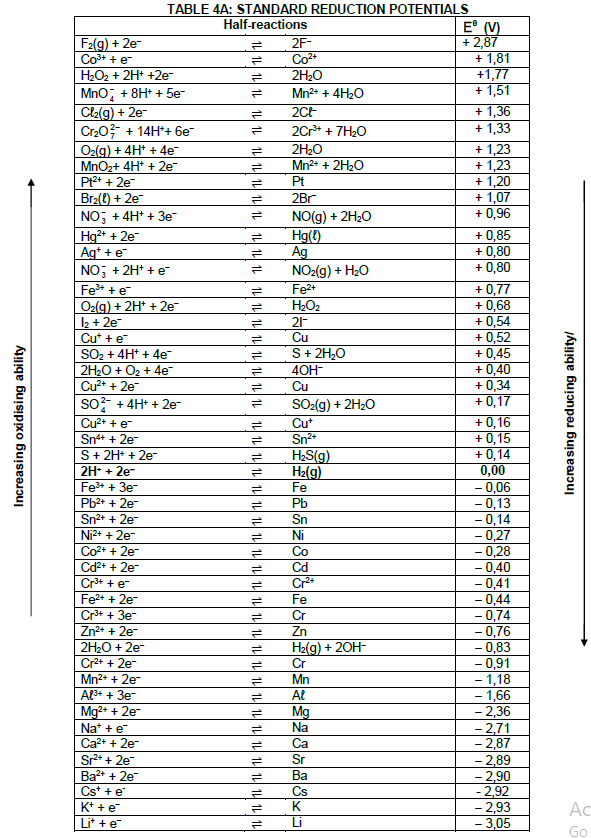
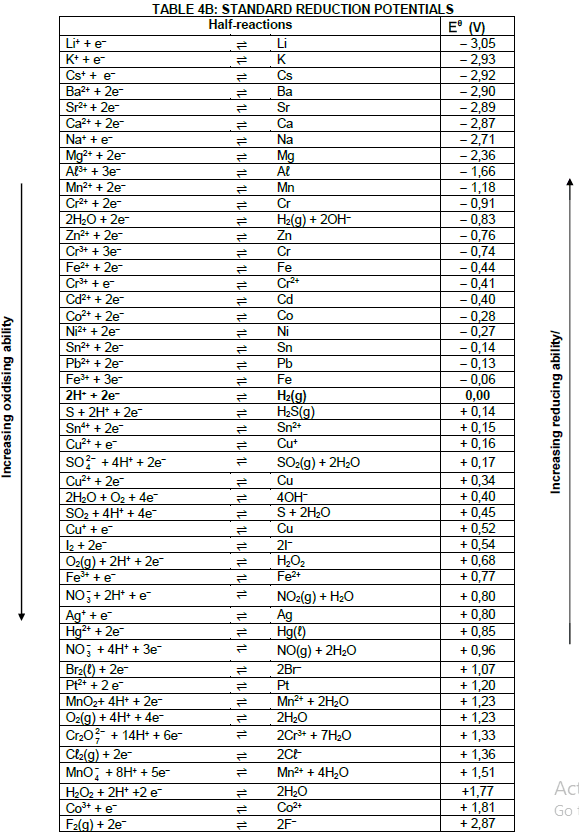
DATA FOR TECHNICAL SCIENCES GRADE 12 PAPER 2
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,01 x 105 Pa |
| Standard temperature | Tθ | 273 K |
| Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
| Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT
| WAVES, SOUND AND LIGHT | |
| v = f λ | T = 1 f |
| Energy | E = hf |
TABLE 3: FORMULAE
| Eθcell= Eθcathode - Eθanode Eθcell = Eθreduction - Eθoxidation Eθcell= Eθoxidising agent - Eθreducing agent |