PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC EXAMS PAST PAPERS AND MEMOS MAY/JUNE 2021
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE EXAMINATIONS
MEMORANDUM
MAY/JUNE 2021
QUESTION 1
1.1 C ✓✓(2)
1.2 D ✓✓ (2)
1.3 C ✓✓ (2)
1.4 B ✓✓ (2)
1.5 D ✓✓ (2)
1.6 C ✓✓ (2)
1.7 B ✓✓ (2)
1.8 B ✓✓ (2)
1.9 A ✓✓ (2)
1.10 B✓✓ (2)
[20]
QUESTION 2
2.1
2.1.1 F (1)
2.1.2 B & F (1)
2.1.3 C (1)
2.2
2.2.1 Haloalkane / alkyl halide(1)
2.2.2 3,5-dibromooctane
Marking criteria:
- Octane
- Dibromo
- Substituents (dibromo) correctly numbered, hyphens, commas correctly used. (3)
2.3
2.3.1 Pentan-3-one
OR
3-pentanone
Marking criteria
- Pentanone/pentanoon
- Correct position of functional group (2)
2.3.2 3-methyl butan-2-one
OR
3-methyl butanone
OR
methyl butanone
OR
3-methyl- 2-butanone (2)
2.4
2.4.1 Hexyl methanoate (2)
2.4.2  (1)
(1)
2.5
2.5.1 Cracking/Elimination (1)
2.5.2 C7H16(2)
2.5.3 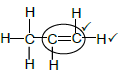
Notes
- Functional group
- Whole structure correct (2)
[19]
QUESTION 3
3.1 Marking guidelines;
If any one of the underlined key phrases in the correct context is omitted, deduct 1 mark
The pressure exerted by a vapour at equilibrium with its liquid in a closed system.(2)
3.2 Functional group/Type of intermolecular forces/Homologous series (1)
3.3 B (1)
3.4 Marking criteria
- State hydrogen bonding in A.
- State dipole-dipole forces in B
- Compare strengths of IMFs
- Compare energies required
- Compound A/butan-1-ol has hydrogen bonding (dipole-dipole and London forces) between molecules.
- Compound B/butan-2-one has dipole-dipole forces (and London forces) between molecules.
- Intermolecular forces in compound A/butan-1-ol are stronger than intermolecular forces in compound B/butan-2-one.
- OR
Intermolecular forces in compound B/butan-2-one are weaker than intermolecular forces in compound A/butan-1-ol. - More energy is needed to overcome/break intermolecular forces in compound A/butan-ol than in compound B/butan-2-one. (4)
3.5
3.5.1 Boiling point (of compound A/butan-1-ol)(1)
3.5.2 Gas (1)
3.5.3 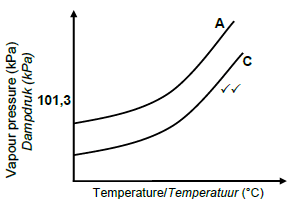
Marking criteria:
- Curve C starts below curve A
- Curve C remains below curve A(2)
Accept
- If C is labelled as B
- If graph below graph A is unlabelled
Note:If both graphs unlabelled: 0 marks
[12]
QUESTION 4
4.1
4.1.1 Heat/sunlight/ultraviolet light/radiation/light(1)
4.1.2 HBr/hydrogen bromide(1)
4.1.3 Hydrolysis(1)
4.1.4 H2O/water
Accept
hydrogen oxide
OR
NaOH/KOH/LiOH/sodium hydroxide/potassium hydroxide/lithium hydroxide (1)
4.1.5 2-bromo propane(2)
4.2 Marking criteria:
(Mark bullets independently)
- React chloroethane with (conc) NaOH or NaOH in ethanol.
- Indicate heat/Δ (on the arrow) or as a reactant in the reaction of chloroethane.
- Correct condensed formula for ethene as product.
- Product NaCℓ in the reaction of chloroethane.
- Product H2O in the reaction of chloroethane.
- React ethene with H2
- Indicate Pt on the arrow of / at the reaction of ethene with H2
- Correct condensed formula of ethane as product.
+ NaOH (in ethanol)
CH3CH2Cℓ + (conc) NaOH → CH2CH2 + NaCℓ + H2O
CH2CH2 + H2 → CH3CH3
Note:
Any additional reactants or products: Deduct one mark per reaction (8)
[14]
QUESTION 5
5.1 NOTE
Give the mark for per unit time only if in context of reaction rate.
ANY ONE
- Change in concentration of products/reactants per (unit) time.
- Change in amount/number of moles/volume/mass of products or reactants per (unit) time.
- Amount/number of moles/volume/mass of products formed/reactants used per (unit) time.
- Rate of change in concentration/amount/number of moles/volume/mass. (2 or 0)(2)
5.2
- Time/tyd
- Volume of gas/CO2/carbon dioxide (in gas syringe)
OR
- Time taken for Aℓ2(CO3)3 to be used up.
Accept
Measure volume of gas/CO2 at regular time intervals.(2)
5.3 Experiment II:
- More (HCℓ) particles per unit volume./More particles with correct orientation.
- More effective collisions per unit time./Higher frequency of effective collisions.
- Higher reaction rate.(3)
OR
Experiment I:
- Less (HCℓ) particles per unit volume.
- Less effective collisions per unit time./Lower frequency of effective collisions.
- Lower reaction rate.
5.4 OPTION 1
ave rate = - Δn
Δt
4,4 x 10-3 = -nr - 0.016
2,5 (0)
n[Aℓ2(CO3)3] = 0,005 (mol)
Marking criteria:
- Substitute average rate and Δt
- Substitute Δn.
- Final answer: 0,005 (mol)
OPTION 2
ave rate = - Δn
Δt
4,4 x 10-3 = Δn
2,5
n[Aℓ2(CO3)3] = 0,016 – 0,011
= 0,005 mol
NOTE
- Accept negative answers when the negative sign in front of the formula is omitted
- Do not penalise if initial and final mole values or time values are swopped.
OPTION 3
With reference to CO2
ave. rate = Δn
Δt
4,4 x 10-3 = Δn
2,5
Δn(CO2) = 0,011 mol
n(CO2) : n(Aℓ2(CO3)3
3 : 1
0,011 : 3,67 x 10-3 mol
n(Aℓ2(CO3)3 left = 0,016 - 3,67 x 10-3 = 1,23 x 10-2 mol
OPTION 4
With reference to HCℓ
ave. rate = Δn
Δt
4,4 x 10-3 = Δn
2,5
Δn(HCℓ) = 0,011 mol
n[Aℓ2(CO3)3] = 0,011 = 0.0018 mol
6
n(Aℓ2(CO3)3 left = 0,016 - 0,0055 = 0,0105 mol
OPTION 5
With reference to AℓCℓ3
ave. rate= Δn
Δt
4,4 x 10-3 = Δn
2,5
Δn(HCℓ) = 0,011 mol
n[Aℓ2(CO3)3] = 0,0055 mol
n[Aℓ2(CO3)3] left = 0,016 – 0,0055 = 0,0105 mol (3)
5.5 Marking criteria
Use mol ratio: n(CO2) : n(Aℓ2(CO3)3) = 3 : 1
Substitute 24 000 cm3∙mol-1/24 dm3∙mol-1 in n = V/VM or in ratio.
Final answer/Finale antwoord: 1 152 cm3 / 1,152 dm3
OPTION 1
n(CO2) = 3n[Aℓ2(CO3)3]
= 3(0,016)
= 0,048 mol
n(CO2) = V
VM
0,048 = V
24000
V(CO2) = 1 152 cm3 (1,152 dm3)
OPTION 2
n(CO2) = 3n[Aℓ2(CO3)3]
= 3(0,016)
= 0,048 mol
1 mol ……………….24 000 cm3
0,048 mol ………….V
V(CO2) =0,048 x 24000
1
= 1 152 cm3 (1,152 dm3) (3)
[13]
QUESTION 6
6.1 (The stage in a chemical reaction when the) rate of forward reaction equals the rate of reverse reaction.
OR
(The stage in a chemical reaction when the) concentrations of reactants and products remain constant. (2)
6.2
6.2.1 X
ANY ONE
- The concentration of products increases (from 0 – 6 min.).
- The concentration of reactants decreases (from 0 – 6 min.).
- No products were present initially.
- The curve begins at zero. (2)
6.2.2 Higher than (1)
6.3 CALCULATIONS USING NUMBER OF MOLES
Marking criteria
- Calculate mol HI: n(HI)ini. = 1(0,5).
- Use mol ratio: 2:1:1 / n(HI) = 2n(H2) = 2n(I2).
- n(H2)equilibrium = n(H2)formed
n(I2)equilibrium= n(I2)formed
Note: If Δn not shown award mark for equal nequilibrium - n((HI)equilibrium = n(HI)initial - n(HI)change
- Divide n(HI)equil & n(H2)equil & n(H2)equil by 0,5 dm3
- Correct Kc expression (formulae in square brackets)
- Substitute 0,04 into Kc expression
- Substitute equilibrium concentrations in Kc expression.
- Final answer 0,07 mol
Range: 0,07 – 0,072 mol
OPTION 1
n(HI) = 1(0,5) = 0,5 mol
| HI | H2 | I2 | |
| Initial quantity (mol) | 0.5 | 0 | 0 |
| Change (mol) | 2x | x | x |
| Quantity at equilibrium (mol) | 0.5- 2x | x | x |
| Equilibrium concentration (mol∙dm-3) | 0.5 - 2x 0.5 | x 0.5 | x 0.5 |
Kc =[H2][I2 ]
[HI]2
0,04 =(x/0.5)(x/0.5)
(0,5 - 2x)2
0,5
x = 0,071 mol
CALCULATIONS USING CONCENTRATION
Marking criteria:
- Use initial c(HI) = 1 mol∙dm-3.
- Use mol ratio: 2 : 1: 1 / n(HI) = 2n(H2) = 2n(I2).
- c(H2)equilibrium = c(H2)formed
c(I2)equilibrium = c(I2)formed
Note: If Δc not shown award mark for equal cequilibrium - c(HI)equilibrium = c(HI)initial - c(HI)change.
- Correct Kc expression (formulae in square brackets).
- Substitution of 0,04 into Kc expression.
- Substitution of equilibrium concentrations into Kc expression.
- Multiply concentration by 0,5 dm3.
- Final answer: 0,07 mol
Range: 0,07 to/tot 0,072 mol
OPTION 2
| HI | H2 | I2 | |
| Initial quantity (mol) | 1 | 0 | 0 |
| Change (mol) | 2x | x | x |
| Equilibrium concentration (mol∙dm-3) | 1-2x | x | x |
Kc =[H2][I2 ]
[HI]2
0,04 = (x)(x)
(1 - 2x)2
x = 0,143 mol∙dm-3
n(I2) = cV
= 0,143 x 0,5
= 0,072 mol (9)
6.4
6.4.1 Both forward and reverse(1)
6.4.2 Positive
- The forward reaction is favoured.
- An increase in temperature favours the endothermic reaction.
- The forward reaction is endothermic(4)
[19]
QUESTION 7
7.1 Standard solution(1)
7.2
7.2.1 Marking criteria
- Any one of the formulae c = m/MV / n = m/M/c = n/V
- Substitution of 40 g∙mol-1 into correct formula.
- Substitution of 0,25 dm3 into correct formula.
- Final answer/Finale antwoord: 0,2 mol∙dm-3
OPTION 1
c = m/MV
= 2
40 x 0.25
= 0,20 mol∙dm-3
OPTION 2
n = m/M
= 2/40
= 0,05 mol
c = n/V
= 0,05
0,25
= 0,20 mol∙dm-3 (4)
7.2.2 POSITIVE MARKING FROM 7.2.1.
OPTION 1
[H3O+][OH-] = 1 x 10-14
[H3O+](0,2) = 1 x 10-14
[H3O+] = 5 x 10-14 mol∙dm-3
pH = -log[H3O+]
= -log(5 x 10-14)
= 13,30
OPTION 2
pOH = -log[OH-]
= -log(0,2)
= 0,6989 (0,7)
pH + pOH = 14
pH = 14 – 0,6989
= 13,30 (4)
7.3 POSITIVE MARKING FROM QUESTION 7.2.
Marking criteria:
- Substitution to calculate n(NaOH).
- Use mol ratio: n(HCℓ)excess: n(NaOH) = 1 : 1.
- Substitute 100 g·mol-1 in n = m/M
- Use mol ratio: n(HCℓ)reacted : n(CaCO3) = 2 : 1.
- n(HCℓ)initial = n(HCℓ) excess + n(HCℓ) reacted
- Substitute 0,05 dm3 to calculate either c(HCℓ)initial or c(HCℓ) reacted
- Final answer: 0,7 mol∙dm-3
Range: 0,70 to 0,90 mol∙dm-3
OPTION 1
n(NaOH)used = cbVb
= 0,2 x 0,025
= 5 x 10-3 mol
OPTION 2
n(NaOH)used = 25 x 2
250 40
= 5 x 10-3 mol
n(HCℓ)excess = n(NaOH) = 5 x 10-3 mol
n(CaCO3) = m/M
= 1.5
100
= 0,015 mol (0,02 mol)
n(HCℓ)reacted= 2n(CaCO3) = 0,03 mol (0,04 mol)
n(HCℓ)ini. = 5 x 10-3 + 0,03
= 0,035 mol (0,045 mol)
c(HCℓ)ini = n/V
= 0,035
0,05
= 0,70 mol∙dm-3 (0,90 mol∙dm-3)
OPTION 3
caVa = na
cbVb nb
ca (0,025) = 1
(0,2)(0,05) 1
ca = c(HCℓ)excess
= 0,1 mol·dm-3
OPTION 4
(NaOH)used = cbVb
= (0,2)(0,025)
= 0,005 mol
n(HCℓ)excess = n(NaOH)
= 0,005 mol
c(HCℓ)excess = 0,005
0,05
= 0,1 mol·dm-3
n(CaCO3) = m/M
= 1.5
100
= 0,015 mol
n(CaCO3) : n(HCℓ) = 1 : 2
n(HCℓ)reacted = 2(0,015)
= 0,03 mol
c(HCℓ)reacted = n/V
= 0,03
0,05
= 0,6 mol·dm-3
c(HCℓ)initial = c(HCℓ)reacted + c(HCℓ)excess
= 0,6 + 0,1
= 0,7 mol·dm-3 (8)
[17]
QUESTION 8
8.1
8.1.1 Gain of electrons. (2 or 0) (2)
8.1.2 2H2O(ℓ) + 2e─ H2(g) + 2OH─(aq)
Ignore phases.
Marking criteria:
- H2(g) + 2OH─(aq) ← 2H2O(ℓ) + 2e─ (2/2)
2H2O(ℓ) + 2e─ ⇌ H2(g) + 2OH─(aq) (½)
H2g) + 2OH─(aq) ⇌ 2H2O(ℓ) + 2e─ (0/2)
2H2O(ℓ) + 2e─ ← H2(g) + 2OH─(aq) (0/2) - Ignore if charge omitted on electron.
- If charge (-) omitted on OH─
Example: 2H2O(ℓ) + 2e─ H2(g) + 2OH(aq) Max.:½ (2)
8.1.3 2Na(s) + 2H2O(ℓ) → H2(g) + 2OH-(aq) + 2Na+(aq) Bal
OR
2Na(s) + 2H2O(ℓ) → H2(g) + 2NaOH(aq) Bal
Ignore phases/.
Marking criteria:
- Reactants Products Balancing
- Ignore double arrows.
- Ignore phases
- Marking rule 6.3.10. (3)
8.1.4 Formation of hydroxide ions / OH- / sodium hydroxide/base/ alkaline/ pH > 7(1)
8.1.5 Cu is a weaker reducing agent than H2 (and OH─) and H2O will not be reduced (to H2 and OH─).
OR
H2 (and OH-) are stronger reducing agent than Cu and H2O will not be reduced(to H2 and OH-). (3)
8.2
8.2.1 Phase separator/boundary/difference(1)
8.2.2 Chemical (energy) to electrical (energy)(1)
8.2.3 OPTION 1
Eθcell = Eθreduction - Eθoxidation
= 0,77 - (-0,13)
Eθcell = 0,90 V
- Notes
Accept any other correct formula from the data sheet - Any other formula using unconventional abbreviations, e.g. Eθcell = EθOA - EθRA followed by correct substitutions:(4)
OPTION 2
Pb(s) → Pb2+(aq) + 2e- 0,13 (V)
2Fe3+(aq) + 2e- → 2Fe2+(aq) 0,77 (V)
Pb2+(aq) + 2Fe3+(aq) → Pb(s) + 2Fe2+(aq) 0,90 V
[17]
QUESTION 9
9.1 Electrolytic (cell)
Cells have a battery/DC power source/ /Electrical energy is converted to chemical energy. (2)
9.2
9.2.1 2Cℓ─ Cℓ2 + 2e─
Notes:
2Cℓ─ ⇌ Cℓ2 + 2e─ (½) Cℓ2 + 2e─ ← 2Cℓ─ (2/2)
Cℓ2 + 2e─ ⇌ 2Cℓ─ (0/2 ) 2Cℓ─ ← Cℓ2 + 2e─ (0/2)
- Ignore if charge omitted on electron.
- If charge (-) omitted on Cℓ─
9.2.2 Aℓ3+ + 3e─ → Aℓ
Notes/Aantekeninge
Aℓ3+ + 3e─ ⇌ Aℓ (½) Aℓ ← Aℓ3+ + 3e─ (2/2)
Aℓ ⇌ Aℓ3+ + 3e─ (0/2) Aℓ3+ + 3e ← Aℓ(0/2)
- Ignore if charge omitted on electron.
- If charge (+) omitted on Aℓ3+
Example: Aℓ3(aq) + 3e─ → Aℓ(s) Max. ½ (2)
9.2.3 Cu/copper(1)
9.3 ANY ONE
- The electrode/carbon/C reacts with oxygen.
- C + O2 → CO2
- Oxidation takes place./Electrons are lost.
- Oxygen corrodes the carbon electrode. (1)
[8]
QUESTION 10
10.1
10.1.1 Sulphur dioxide/SO2(1)
10.1.2 Sulphur trioxide/SO3(1)
10.1.3 Vanadium pentoxide/V2O5/ Vanadium(V) oxide(1)
10.1.4 H2SO4 + 2NH3 → (NH4)2SO4 bal
Marking guidelines:
- Reactants Products Balancing
- Ignore → and phases
- Marking rule 6.3.10 (3)
10.2
10.2.1 The ratio of nitrogen (N), phosphorous (P) and potassium (K) in a fertiliser./The ratio of the primary nutrients (1)
10.2.2 OPTION 1
Mass N in 4 kg NH4NO3 / Massa N in 4 kg NH4NO3
m(N) = 28/80 x 4
= 1,4 kg
m(K) = 2m(N)
= 2,8 kg
m(P) = 3m(N)
= 4,2 kg
m(fertiliser) = 1,4 + 2,8 + 4,2
= 8,4 kg
OPTION 2
Mass N in 4 kg NH4NO3 / Massa N in 4 kg NH4NO3
m(N) = 28/80 x 4
= 1,4 kg
N : P : K
1 : 3 : 2
∴ m(fertiliser) = (6)(1,4)
= 8,4 kg
OPTION 3
% N = (2)(14) x 100 = 35%
80
Nitrogen in 4 kg = 35% of 4 = 1,4 kg
N : P : K
1 : 3 : 2
1,4 : 4,2 : 2,8
Total mass of fertiliser = 1,4 + 4,2 + 2,8
= 8,4 kg (4)
[11]
TOTAL: 150