Technical Sciences Paper 2 Grade 12 Questions - NSC Past Papers And Memos September 2020 Preparatory Examinations
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
Read the following instructions carefully before answering the questions.
- Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- You may use a non-programmable calculator.
- Leave ONE line between two sub-questions for example between QUESTION 2.1 and QUESTION 2.2.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers
(1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 Which ONE of the following organic compounds does NOT contain a carbonyl group?
- Aldehydes
- Alcohols
- Ketones
- Esters (2)
1.2 Which ONE of the following general formulae represents alkynes?
- CnH2n-2
- CnH2n-1
- CnH2n
- CnH2n+2 (2)
1.3 Which ONE of the following pairs of reactants can be used to prepare the ester ethyl butanoate in the laboratory?
- Ethanal and butanol
- Ethanoic acid and butanol
- Ethanol and butanoic acid
- Ethanal and butanoic acid (2)
1.4 Which ONE of the following compounds represents a ketone?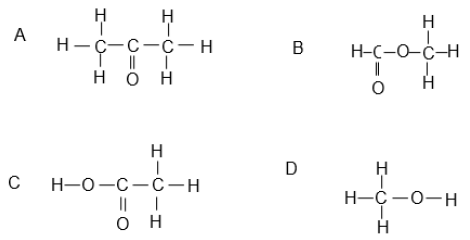
(2)
1.5 The structural formula of a haloalkane is represented below.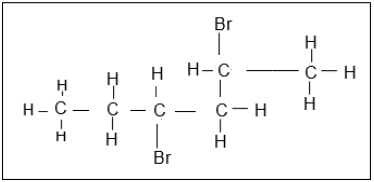
Which ONE of the following descriptions is the correct IUPAC NAME for this organic molecule?
- 3,5-dibromohexane
- 4-bromo-5-bromo-5-methylpentane
- 2,4-dibromohexane
- 2-bromo-1-bromo-1-methylpentane (2)
1.6 A ray of light strikes a glass block at an angle θ, as shown below. The ray passes through the glass block and emerges on the opposite side.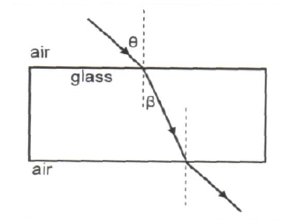
At what angle to the normal does the ray emerge from the glass block?
- β
- θ
- 90º – θ
- 90º – β (2)
1.7 When total internal reflection takes place, the size of the angle of reflection is …
- smaller than the angle of incidence.
- greater than the angle of incidence.
- the same as the angle of incidence.
- the same as the critical angle. (2)
1.8 The magnification of a convex lens is 2. The distance of the object from the optical centre is 30 mm. The image distance is …
- 15 mm.
- 28 mm.
- 30 mm.
- 60 mm. (2)
1.9 The phenomenon by which a light ray strikes a surface and goes back into the medium of incidence is known as …
- reflection.
- diffraction.
- refraction.
- dispersion. (2)
1.10 If an object is placed between the focal point and the convex lens, the image formed will be …
- enlarged, inverted and virtual.
- enlarged, upright and virtual.
- smaller, inverted and real.
- smaller, upright and real. (2)
[20]
QUESTION 2 (Start on a NEW page.)
Organic chemistry is the chemistry of organic molecules divided into homologous series which are identified by the functional groups. It is this branch of chemistry that is applied in the industry and in medicine. Pharmaceuticals used in cancer treatment and other conditions follow organic synthesis.
2.1 Explain the term homologous series. (2)
2.2 Define the term functional group. (2)
2.3 Study the organic compounds listed in the table below, labelled A‒F and answer the questions that follow.
| A | B | C |
| But-2-ene | 1-chlorobutan-2-one | Propan-1-ol |
| D | E | F |
| 3-bromo-5- methylhexene | 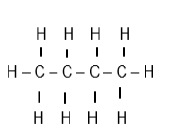 | 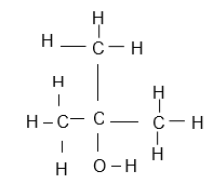 |
Write down the LETTER that represents:
2.3.1 An alkane (1)
2.3.2 A primary alcohol (1)
2.4 Write down the IUPAC name of structures:
2.4.1 E (2)
2.4.2 F (2)
2.5 Draw the structural formula of:
2.5.1 A (2)
2.5.2 C (2)
2.5.3 An isomer of E (2)
2.6 Define the term positional isomer in words. (2)
2.7 To which homologous series does compound B belong? (1)
2.8 Draw the structural formula of the functional group of compound B. (2)
[21]
QUESTION 3 (Start on a NEW page.)
Rubber is a naturally occurring compound. The diene, 2-methyl-1,3-butandiene, is one of the repeating units found in rubber.
Over 20 million families depend on rubber cultivation for their livelihood. Tens of thousands of hectares of tropical forests have been cleared to make way for rubber plantations.
Chemists have been able to combine other dienes to obtain synthetic rubbers. Some rubber products include latex products such as hand gloves, raincoats and other products used in the fight against HIV/Aids and COVID-19.
The world’s largest use of rubber is in the production of tyres, and most tyres contain both natural rubber, which withstands heat better, and one or more kinds of synthetic rubber.
3.1 Define the term polymer in words. (2)
3.2 Is but-2-ene an example of a saturated or an unsaturated hydrocarbon? Give a reason for the answer. (3)
3.3 Write down the structural formula of 2-methylbutane. (2)
3.4 With regard to the environment, name TWO disadvantages of rubber and the production of rubber. (2)
3.5 With regard to human life, name TWO benefits of rubber and the production of rubber. (4)
[13]
QUESTION 4 (Start on a NEW page.)
In the table below FIVE alcohols, represented by the letters A–E, are listed.
| A | Methanol | B | Ethanol |
| C | Propan-1-ol | D | Butan-2-nol |
| E | 2-methylpropan-2-ol |
4.1 Write down the letter that represents a secondary alcohol from the above list. (2)
4.2 Letter E represents 2-methylpropan-2-ol. Write down the following for this alcohol:
4.2.1 The structural formula (2)
4.2.2 The letter of an alcohol that represents its structural isomer (2)
4.3 Viscosity is a measure of a fluid's resistance to flow. Learners conduct an investigation to compare the viscosities of the first three alcohols (A–C) in the above table. They use the apparatus shown below.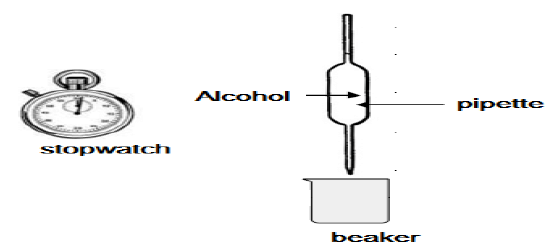
The learners use a stopwatch to measure the time it takes a FIXED VOLUME of each of the alcohols to flow from the pipette. They record this flow time, which is an indication of the viscosity of each alcohol, as given in the table below.
| ALCOHOL | FLOW TIME (s) | |
| A | Methanol | 4,0 |
| B | Ethanol | 7,9 |
| C | Propan-1-ol | 14,3 |
4.3.1 Which ONE of the alcohols A, B, or C has the highest viscosity? Use the data in the table to give a reason for the answer. (2)
4.3.2 Refer to the intermolecular forces of the three alcohols A, B and C to explain the trend in viscosities as shown in the table. (3)
4.3.3 Lubricants reduce friction. Which ONE of alcohols, A, B or C, will be the best lubricant? Explain your answer. (3)
4.3.4 Define the term vapour pressure in words. (2)
4.4 Which ONE of 2-methylpropan-2-ol and butan-2-ol has a higher viscosity? Explain the answer. (3)
[19]
QUESTION 5 (Start on a NEW page.)
In the flow diagram below X, Y and Z represent three different types of organic reactions. P represents an organic compound.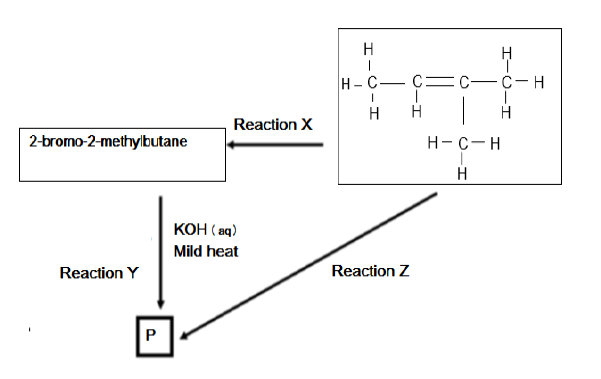
5.1 Name the type of reaction represented by reaction X. (2)
5.2 State TWO reaction conditions required for reaction X to take place. (2)
5.3 Reaction Y represents a substitution reaction. Write down the structural formula of compound P formed in the reaction. (2)
5.4 Apart from the organic reactant, write down the NAME or FORMULA of the other reactant needed in reaction Z (2)
5.5 Write down a balanced chemical reaction for the formation of compound P using structural formulae. (4)
5.6 Name the type of reaction represented by reaction Z. (2)
5.7 A car engine at a Shell Garage in Willowvale was running when a debate started between learners of a Technical Sciences class in a particular secondary school in that area. They noticed smoke coming out of the exhaust system and liquid droplets at the back of the exhaust system. One learner claimed that hexane was burning inside. They eventually reached an agreement about their observations.
5.7.1 Give the names of the products of combustion of hexane. (2)
5.7.2 Write down a balanced equation for the complete combustion of hexane. (4)
[20]
QUESTION 6 (Start on a NEW page.)
Hexanoic acid is responsible for the unique odour associated with goats. When it reacts with alcohol X, ethylhexanoate, which is used commercially as a fruit flavour is formed.
Learners set up the apparatus shown below to prepare ethylhexanoate in a laboratory.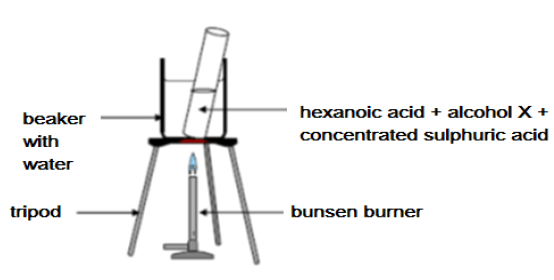
6.1 Write down the IUPAC name of alcohol X. (2)
6.2 What is the role of the sulphuric acid in the above reaction? (2)
6.3 Use structural formulae to write down a balanced equation for the preparation of ethyl hexanoate. (4)
6.4 Give ONE reason why the test tube and its contents are heated in a water bath and not directly over the flame. (2)
6.5 Write down ONE use of esters in the food manufacturing industry. (2)
[12]
QUESTION 7 (Start on a NEW page.)
Below is part of a student’s revision notes about waves. The notes contain several scientific errors.
WAVES
|
7.1 The above statements are incorrect. Identify the errors and show how each error can be corrected. (6)
7.2 The diagrams below show the refraction of light as it passes from air into different media, benzene, diamond and water.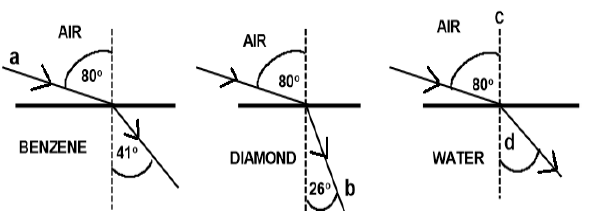
Write down the names for the following:
7.2.1 Light ray a (1)
7.2.2 Light ray b (1)
7.2.3 Dashed line c (1)
7.2.4 The angle labelled d (1)
7.3 Define the term refraction in words. (2)
[12]
QUESTION 8 (Start on a NEW page.)
The diagram below shows a ray of light travelling inside a denser medium and did not leave the denser medium to enter the less dense medium. This is a phenomenon in waves.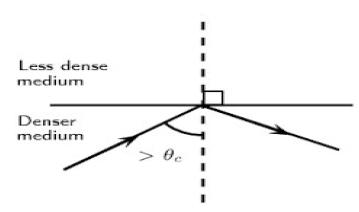
8.1 Name the phenomenon illustrated in the diagram above. (1)
8.2 Describe this phenomenon in words. (2)
8.3 What TWO conditions must be satisfied in order for the light ray to behave as it does in the diagram above? (2)
8.4 Give TWO essential applications of this phenomenon. (2)
8.5 Define the term critical angle in words. (2)
8.6 State the Law of Reflection in words. (2)
8.7
The same light ray is now passed in a similar medium as illustrated above but another phenomenon is demonstrated as is observed in this diagram.
8.7.1 Name the phenomenon demonstrated in this diagram. (1)
8.7.2 Describe this phenomenon that is demonstrated in the diagram. (2)
8.8 Sipho uses a magnifying glass with a focal length of 4 cm to view an ant. He finds that he gets a clear image of the ant when it is 2,5 cm away from the lens. The ant is 1 cm in size.
8.8.1 What type of lens does a magnifying glass represents? (1)
8.8.2 Define focal length. (2)
8.8.3 Give THREE properties of the image formed if ray diagrams were used. (3)
[20]
QUESTION 9 (Start on a NEW page.)
Albert Einstein, Geiger and Gamma suggest that light can act as a particle or as a wave. The diagram below represents radiations of the electromagnetic spectrum.
| Infrared | Radio waves | X-ray | Microwave | Gamma rays | Ultraviolet rays | Visible light |
9.1 Arrange the spectrum in order of increasing frequency. (2)
9.2 In which radiation will a photon have the highest energy? (1)
9.3 Explain the answer to QUESTION 9.2. (2)
9.4 Define the term electromagnetic waves in words. (2)
9.5 Calculate the energy of a photon of an electromagnetic radiation with a wavelength of 540 nm. (5)
9.6 How will the energy of a photon with a wavelength of 520 nm compare with that calculated in QUESTION 9.5? (Write only GREATER THAN, SMALLER THAN or EQUAL TO.) (1)
[13]
TOTAL: 150
DATA TABLES FOR TECHNICAL SCIENCE GRADE 12
PAPER 2
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,01 x 105 Pa |
Standard temperature | Tθ | 273 K |
Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT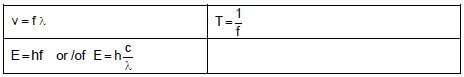
TABLE 3: ELECTROCHEMISTRY
Eθcell = Eθcathode - Eθanode |
TABLE 3: THE PERIODIC TABLE OF ELEMENTS