Physical Science Paper 2 Grade 12 Memorandum - NSC Past Papers And Memos September 2020 Preparatory Examinations
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupMEMORANDUM
QUESTION 1
1.1 D (2)
1.2 C (2)
1.3 A (2)
1.4 A (2)
1.5 C (2)
1.6 B (2)
1.7 B (2)
1.8 D (2)
1.9 D (2)
1.10 B (2) [20]
QUESTION 2
2.1
- A series of organic compounds that can be described by the same general formula
OR
A series of organic compounds in which members differ by the number of – CH2 units (2)
2.2 A compound that contains carbon and hydrogen (atoms) only (2)
2.3.1 CnH2n+2 (1)
2.3.2 4-ethyl -2,2-dimethyl hexane
Marking criteria
- Hexane
- Methyl/Metiel and
- whole name correct 3/3
Deduct 1 mark for any error, hyphens omitted, incorrect sequence etc (3)
2.4.1 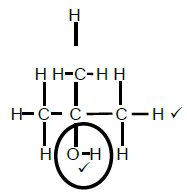 (2)
(2)
Marking criteria
- Hexane
- Methyl and ethyl
- whole name correct 3/3
Deduct 1 mark for any error, hyphens omitted, incorrect sequence etc
2.4.2 Butan-2-one OR 2-butanone (2)
Marking criteria
- Functional group and correct position g pent-2-one (1/2)
- Whole name correct (2/2)
2.4.3
- The functional group can only be in position 1
OR
Side chain / branch / methyl group can only be in position 2 (1)
2.5.1 Polymerisation (1)
2.5.2 Contains single bonds only (1 or 0) (1)
2.5.3 Use in plastics (toy cars) (1)
[16]
QUESTION 3
3.1 The temperature at which the vapour pressure of a liquid equals the atmospheric/external pressure. (2)
3.2 To ensure a fair test /To ensure there is one independent variable (1)
3.3
- Hexane has London forces (only)
Pentanal has dipole-dipole forces (and London forces) - Dipole-dipole forces are stronger ( than the london forces in hexane)
OR London forces are weaker (than the Dipole-dipole forces in pentanal) - More energy is required to overcome the intermolecular /Dipole-dipole forces in Pentanal (4)
3.4 Higher than (1)
3.5
- Chain isomer of pentan-2-ol (2-methylbutan-2-ol) has a shorter chain length/ smaller surface area than pentan-2-ol
- London forces in the isomer of pentan-2-ol (2-methylbutan-2-ol) will be weaker (than that of pentan-2-ol).
OR - Pentan-2-ol has a larger chain length/ surface area than its chain isomer
- London forces in butan-2-ol is stronger (than that of the isomer of pentan-2-ol (2-methylbutan-2-ol) (3)
3.6 2 C6H14 + 19 O2 → 12 CO2 + 14 H2O
Marking criteria
- Reactants
- Products
- Balancing (3)
[14]
QUESTION 4
4.1.1 Substitution /Hydrolysis (1)
4.1.2 Butan-2-ol OR/OF 2-butanol (2)
4.2 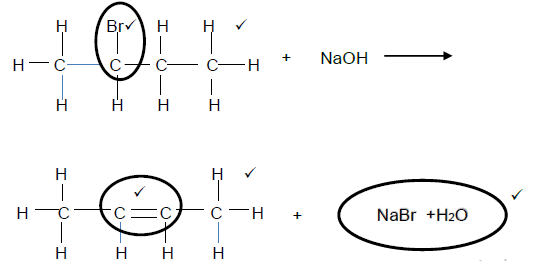 (6)
(6)
Balancing
Marking criteria
For organic reagents
- Whole structure correct(2/2)
- Functional group correct (1/2)
4.3
4.3.1 Hydrogenation (1)
4.3.2 Platinum/Palladium/Nickel (1)
4.3.3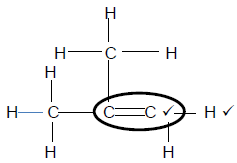
Marking criteria
For organic reagents
- Whole structure correct (2/2)
- Functional group correct (1/2) (2)
4.4
4.4.1 Esterification/Condensation (1)
4.4.2 Heat/Add a catalyst/H2SO4 (1)
4.4.3 Water/H2O (1)
4.4.4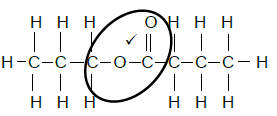
Marking criteria
- Whole structure correct (2/2)
- Functional group correct (1/2)
- Name correct (2/2)
Propyl butanoate (4)
[20]
QUESTION 5
5.1 The change in concentration of reactants or products per unit time.(2)
5.2 Carbon dioxide/CO2 (1)
5.3 Stopwatch (1)
5.4
- Rate = -Δ m/Δt
Rate= –(200 -184,80) / (5 – 0)
Rate = 3,04 (g∙min-1)
Accept:
Rate = (184,80 –200) / (5 – 0) Rate = (200 –184,80) /(5 – 0)
= - 3,04 (g∙min-1) Rate = 3,04 (g∙min-1) (3)
5.5
- mCO2 produced = 200- 184,80
= 15.2 g
nCO2 = m/M
= 15,2 /44
= 0,35 mol
nCaCO3 reacting = 0.35 mol
= 0.35 x 100
= 35 g
Marking guidelines
- Mass of CO2
- Formula n = m/M
- Substitution of 44 into n = m/M
- Use of ratio CaCO3: CO2 (1:1)
- Substitution of 100 into n = m/M
- Final answer
(Ratio) mCaCO3 reacting= n.M (6)
5.6.1 Temperature (1)
5.6.2 B (1)
5.6.3
- Higher temperature increases average kinetic energy of the particles increases.
- More particles have sufficient kinetic energy (to collide effectively) / More particles have Ek greater or equal to Ea
- More effective collisions per unit time (3)
5.7.1
- What effect will the concentration (of a substance) have on the rate of reaction?
OR - What is the relationship between reaction rate and concentration?
OR - How does concentration affect reaction rate?
Marking criteria
For organic reagents
- Independent variable and dependent variable correct
- In the form of a question (2)
5.7.2 LOWER THAN (1)
5.7.3 EQUAL TO(1)
5.8 The same amount of CaCO3 (the limiting reagent) is used in both experiments (2)
[24]
QUESTION 6
6.1 Reaction in which products can be converted back to reactants (2)
6.2 FORWARD REACTION(1)
6.3 No
- The rate of forward reaction is equal to the rate of reverse reaction /Reaction reached equilibrium (3)
6.4.1 Increases (1)
6.4.2 Remains the same (1)
6.4.3 Increases (1)
6.5
- The amount of HI remains constant.
- The volume decreases
- The concentration increases according to c = n/V (2)
6.6.1 High yield Kc is large OR Kc >1 (2)
6.6.2 EXOTHERMIC
- The value Kc decreases with an increase in temperature.
- As temperature increases, the [prdoducts] decreases
- Reverse reaction is favoured by an increase in temperature
OR - The value Kc increases with a decrease in temperature.
- As temperature decreases the [products] increases
- Forward reaction is favoured by a decrease in temperature (4)
6.7.1
- Kc = [HI]2/[H2].[I2]
50,3 = [HI]2/(0.46)(0,39)
[HI] = 3 mol.dm-3 (4)
6.7.2 POSITIVE MARKING FROM 6.7.1
OPTION 1 : CALCULATIONS USING NUMBER OF MOLES
Marking Criteria for Mole Option
- Multiplication/ van c equilibrium by/ met 0,5 dm3 for/ vir I2 ,H2 and HI
- Calculations of moles of HI reacting
- Using mole ratio 2 n(HI) = n(H2) = n (I2) reacting
- Calculation of (H2) initial and n (I2) initial (Δn + nequilibrium)
- Multiplication / van n (I2) by 2 to find n (HI) theoretical
- Substitution into Yield = nproduced x 100
- Final answer (RANGE : 78,95% – 79,37%)
CALCULATION USING MOLES
H2 | I2 | 2HI | |
ni | 0,98 | 0,945 | 0 |
Δn | 0,75 | 0,75 Ratio | 1,5 |
nequilibrium | 0,23 | 0,195 | 1,5 (x 0,5 dm3) |
cequilibrium | 0.46 | 0,39 | 3 |
I2 is the limiting reagent
n (HI) theoretical = 2 (n I2 initial) = 2 x (0,945) = 1,89 mol
% Yield = nproduced X 100 = 1,5/1,89 x 100 = 79,37 %
H2 used as limiting reagent Max 3/7
OPTION 2: CALCULATION USING CONCENTRATIONS
Marking Criteria for concentration Option
- Calculations of cHI reacting
- Using mole ratio 2 c(HI) = c(H2) = c(N2) reacting
- Calculation of cH2 initial and cI2 initial (Δc + cequilibrium )
- Multiplication of cI2 by 2 to find vind nHI theoretical
- Substitution into Yield = n
HIproduced x 100 - Final answer
(RANGE: 78,95% -79,37%)
H2 | I2 | 2HI | |
ci | 1,96 | 1,89 | 0 |
Δc | 1,5 | 1,5 (Ratio) | 3 (cHI equil) |
cequilibrium | 0.46 | 0,39 | 3 |
I2 is the limiting reagent
C (HI) theoretical = 2 x 1,89 = 3,78 mol.dm-3
% Yield = cproduced x 100 =3/3,78 x 100 =79,37% (7)
H2 used as limiting reagent Max 3/7
[28]
QUESTION 7
7.1.1 An acid is a substance that donates protons /H+ions (2)
7.1.2 H2PO4 (1)
7.1.3
- Reaction I : Reverse reaction it accepts a proton (H+) / acts as a base
- Reaction II: Foward reaction donates a proton (H+)/ act as an acid. (2)
7.1.4 HPO42
- The conjugate base of a weak acid
- lower Ka value is the stronger base (3)
7.2.1 Reaction of a salt with water (2)
7.2.2 C2HO4 (2)
7.2.3 (Excess) OH- ions/hydroxide ions are produced (2)
7.3.1 A strong base undergoes complete ionisation /disociation (2)
7.3.2 | OPTION 1 / OPSIE 1 | OPTION 2 / OPSIE 2 | |
Kw = [H3O+][OH-] | pOH = -log[OH-] | ||
1x10-14 = [H3O+](0,5) | pOH = - log (0,5) | ||
[H3O+] = 2x10-14 mol∙dm-3 | pOH = 0,30 | ||
pH = -log[H3O+] | pH + pOH = 14 | ||
pH = - log(2x10-14) | pH + 0,30 = 14 | ||
pH = 13,70 | pH = 13,70 | (5) |
7.3.3 OPTION 1
- caVa = na
cbVb nb
ca(25) = 1
(0,5)(24) 2
ca = 0,24 mol∙dm-3
c = m
MV
0,24 = 7,56
(90+18x)(0,25)
x = 2
OPTION 2 :
Marking guideline
- Substitution of 0,5 and 24/100 in n = cV
- Use of mole ratio in Acid: Base 1:2
- Calculating number of moles of acid in original solution
- Use of 90 in m = nM
- Calculation of mass of water of crystallization in original solution
- Calculating ration of nWater/nAcid
- Final answer
n NaOH reacting = cV= 0.5 x 24/1000 = 0.012 mol
noxalic acid reacting = ½ x 0.012 mol = 0,006 mol
noxalic acid in original solution = 250/25 x 0,006
= 0,06 mol
moxalic acid in original solution = nM = 0,06 x 90
= 5,4 g
m H2O of crystallisation in original solution = 7,56 - 5,4
= 2.16 g
n H2O crystalllisation = 2,16 /18 = 0,12 mol
x = 0,12/0,06 = 2 (7)
[28]
TOTAL 150