Technical Sciences P2 Grade 12 Questions - NSC Exams Past Papers and Memos September 2019 Preparatory Examinations
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
Read the following instructions carefully before answering the questions.
- Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- You may use a non-programmable calculator.
- Leave ONE line between two sub-questions for example between QUESTION 2.1 and QUESTION 2.2.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required. 10. Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 The organic compound below is an example of a …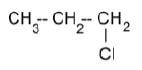
- primary haloalkane.
- secondary haloalkane.
- tertiary haloalkane.
- branched alkane. (2)
1.2 The IUPAC name of the following compound is …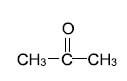
- propanone.
- propanal.
- propanoic acid.
- propanol. (2)
1.3 Ethene can be converted into other carbon-containing compounds using the reactants shown in the following flow chart.
Compounds X and Y are respectively:
X | Y | |
A | bromoethane | methanol |
B | bromoethane | ethanol |
C | bromoethene | ethanoic acid |
D | bromoethene | ethane hydroxide |
(2)
1.4 In which ONE of the following options are the three compounds listed in increasing order of vapour pressure?
- propanoic acid, pentane, butan-1-ol
- propanoic acid, butan-1-ol, pentane
- pentane, butan-1-ol, propanoic acid
- butan-1-ol, propanoic acid, pentane (2)
1.5 An electrochemical cell is to be set up to plate a nickel object with silver. Which ONE of the combinations below CORRECTLY shows the metal used for the positive electrode and the electrolytic solution in the electrochemical cell?
METAL USED FOR POSITIVE ELECTRODE | ELECTROLYTE SOLUTION | |
A | silver | silver nitrate |
B | silver | nickel sulphate |
C | nickel | silver nitrate |
D | nickel | nickel sulphate |
(2)
1.6 The cell notation for a standard Zn–Cu electrochemical cell is:
- Cu2+(aq) / Cu(s) // Zn(s) / Zn2+(aq)
- Zn(s) / Zn2+(aq) // Cu2+(aq) / Cu(s)
- Cu(s) / Zn2+(aq) // Cu2+(aq) / Zn(s)
- Zn(s) / Zn2+(aq) // Cu(s) / Cu2+(aq) (2)
1.7 Which ONE of the following properties demonstrates the wave nature of light?
- Light can be refracted
- Light travels at a speed of 3 x 108 m.s-1
- White light can be separated into a number of different colours
- Light is diffracted after passing through a narrow slit (2)
1.8 A ray of light strikes a rectangular Perspex block so that the angle between the ray and the side of the block is 40°, as shown in the
diagram.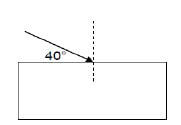
Which ONE of the statements below is correct?
- Angle of incidence is 50°
- Angle of refraction is 40°
- Angle of incidence is 40°
- Angle of refraction is 50° (2)
1.9 A ray of light falls obliquely on the centre of the straight edge of a semi circular glass slab. Which ONE of the following diagrams represents the path of the ray through the slab? 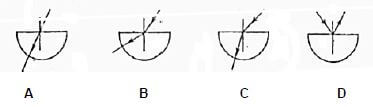 (2)
(2)
1.10 Optical fibres are used in telecommunication because light can be …
- externally reflected.
- internally reflected.
- externally refracted.
- internally refracted. (2) [20]
QUESTION 2 (Start on a NEW page.)
Organic chemistry is the chemistry of organic molecules divided into homologous series which are identified by the functional groups.
2.1 Define the term homologous series. (2)
2.2 Study the organic molecules listed below.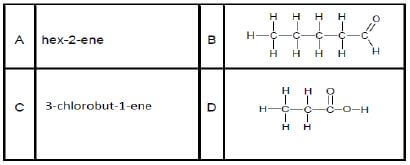
2.2.1 Define the term isomers in words. (2)
2.2.2 Draw the structural formula of a positional isomer of A. (2)
2.2.3 Write down the structural formula of the functional group of B and next to it write down the name of the homologous series to which B belongs. (2)
2.2.4 Draw the structural formula of compound C. (2)
2.2.5 Write down the structural formula of ONE functional isomer of compound D. (2)
2.3 The diagram below shows the structural formula for polyethylene. This is the industrial organic product used in the preparation of plastics.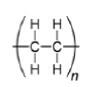
2.3.1 Describe the term monomer. (2)
2.3.2 Write down the IUPAC name of the monomer that formed polyethylene. (2)
2.3.4 Give TWO uses of polyethylene. (2) [18]
QUESTION 3 (Start on a NEW page.)
The table below shows the vapour pressures of various organic compounds at 25 °C.
Compound | Molar mass (g mol-1) | Vapour pressure (X 102 Pa) |
Pentane | 72 | 573,0 |
Hexane | 86 | 160,0 |
Heptane | 100 | 48,0 |
Propan -1-ol | 60 | 21,0 |
Propan -2 ol | 60 | 44,0 |
Butan -1 ol | 74 | 6,2 |
Butan -2 ol | 74 | 18,3 |
Pentan -1-ol | 88 | 2,2 |
Pentan -2- ol | 88 | 8,04 |
Ethanoic acid | 60 | 15,3 |
Propanone | 58 | 240,0 |
3.1 Write down the general formula of the homologous series to which heptane belongs. (1)
3.2 Draw the structural formula of propanone. (2)
3.3 Give the IUPAC name of an isomer of propanone. (2)
3.4 From the table above, write down the name of the intermolecular forces involved in the following:
3.4.1 Alcohols (1)
3.4.2 Alkanes (1)
3.5 State and explain the relationship between vapour pressure and the strength of intermolecular forces. (2)
3.6 Which compound will have the higher boiling point?
Ethanoic acid OR Propan-1-ol
Refer to intermolecular forces and energy in giving reasons for your answer to this difference. (4) [13]
QUESTION 4 (Start on a NEW page.)
The flow diagram below represents a series of organic reactions that took place.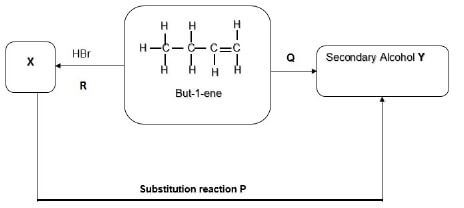
4.1 Name the type of organic reaction represented by reaction Q in the diagram. (1)
4.2 Give the reaction condition(s) required for reaction P which is the hydrohalogenation reaction that forms compound X to take place. (2)
4.3 Write down the IUPAC name of compound X. (2)
4.4 Using structural formulae, write down an equation that represents reaction R which is hydrohalogenation. (3)
4.5 Draw the structural formula of the product Y and give its IUPAC name. (3)
4.6 Write down a balanced equation for the complete combustion of butene (C4H8) in oxygen. (3)
4.7 Give the name of the substitution reaction represented as reaction P. (2) [16]
QUESTION 5 (Start on a NEW page.)
In the electrolytic cell, represented below, two CARBON RODS are used as electrodes and a concentrated copper (II) chloride solution is used as an electrolyte.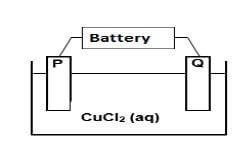
When the cell is functioning, a gas is released at electrode P, whilst electrode Q is coated with a reddish brown layer.
5.1 Define the term electrolyte. (2)
5.2 Give TWO standard conditions for the electrolytic cell when it is in operation. (2)
5.3 Write down a half-reaction to explain the observation made at:
5.3.1 Electrode P (2)
5.3.2 Electrode Q (2)
5.4 What energy conversion is taking place in this cell? (2)
5.5 Which electrode, P or Q, is the cathode? Give a reason for the answer. (2)
5.6 The carbon rods in the above cell are now replaced with COPPER RODS. The following observations are made at electrode P:
- No gas is released
- Its surface appears rough and eroded
5.6.1 Refer to the RELATIVE STRENGTHS OF REDUCING AGENTS to explain this observation. (3)
5.6.2 Will this electrolytic cell operation be a “spontaneous” or “non spontaneous” process? (1) [16]
QUESTION 6 (Start on a NEW page.)
The potential difference of a galvanic cell is measured experimentally by learners. Learners decided to further COMPARE the experimental as well as the calculated potential difference value.
They set up the galvanic cell shown below. 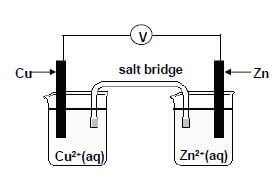
The voltmeter measures an initial reading of 0,9 V.
6.1 Write down the energy conversion that takes place in this cell. (2)
6.2 Which section of the cell will be responsible for electron flow when the cell is in operation? (2)
6.3 State ONE function of the salt bridge other than completing the circuit. (2)
6.4 Write down the half-reaction that takes place at the anode. (2)
6.5 In which direction will the electrons in the external circuit flow when this cell delivers a current? Write down only from Cu to Zn or from Zn to Cu. (1)
6.6 Write down the balanced net (overall) cell reaction. (3)
6.7 Use the Table of Standard Reduction Potential to calculate the initial potential difference (emf) of the above cell at STANDARD CONDITIONS. (4)
6.8 From the results obtained the learners conclude that the measured potential difference differs from the calculated potential difference. Give possible reasons for this difference in values. (3)
6.9 South Africa is experiencing an electricity crisis. There is an alarming shortage of coal which used to be a traditional source of electricity generation in the country. Eskom’s grid is on the law and hence the alternative forms of energy. There are noticeable wind farms in most parts of the country as a form of renewable energy. The most popular type of these forms of renewable energy is photo-voltaic which uses silicon cells that traps radiant energy from the sun hence they are commonly known as solar panels. Global warming is also another universal crisis that can be addressed by use of renewable energies as form of electricity.
6.9.1 Explain TWO environmental threats when solar panels are installed during the construction stage. (2)
6.9.2 What are the economic advantages of using solar panels? (2) [23]
QUESTION 7 (Start on a NEW page.)
‘A diamond is judged by four distinct factors that combine in a number of ways to arrive at its value.’ These are called the 4 C’s and are as follows:
- Carat weight
- Clarity
- Colour
- Cut
Of all the 4 C’s, cut is the one most directly influenced by man. The other three are dictated by nature. The cut or make of a diamond will dramatically influence its fire and sparkle, for it is the cutters skill that releases its beauty.” Geology 306 shows the ray path inside the diamond crystal.
Diagrams A and B below illustrate two different cuts of diamond and the way each cut handles light.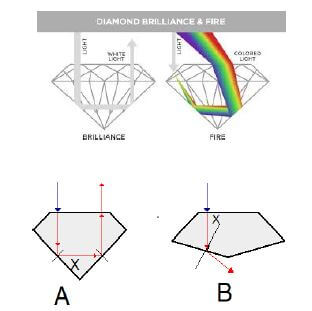
7.1 State the Law of Reflection. (2)
7.2 Identify the following:
7.2.1 Phenomenon illustrated in diagram A (1)
7.2.2 Phenomenon illustrated in diagram B (1)
7.3 Give TWO fundamental applications of the phenomenon illustrated in diagram A above. (2)
7.4 What TWO conditions must be satisfied in order for light to behave as it does in diagram A? (2)
7.5 The critical angle for diamond is 24°.
7.5.1 Define the term critical angle. (2)
7.5.2 Give a possible value for angle X in diagram B. Choose your answer from the values given below.
| 22° | 24° | 26° |
(2)
7.6 Mr I.A.M. Cheap is shopping for a diamond for his wife. Which cut (A or B) do you think his wife would prefer? Give a clear reason for your choice. (2)
7.7 In the diagram “Diamond Brilliance & Fire” above we noticed that white light is changing into the seven primary colours of the spectrum.
7.7.1 Define the phenomenon of these seven colours of the spectrum. (2)
7.7.2 Which colour is refracted the least in the spectrum? (1)
7.7.3 Explain your answer to QUESTION 7.7.2 above in terms of the frequency of light. (2) [19]
QUESTION 8 (Start on a NEW page.)
The diagram below shows the path of a ray of light through one corner of a cube of ice.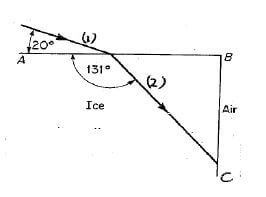
8.1 Define the term refraction. (2)
8.2 Give the names for the rays labelled 1 and 2 in the above diagram. (2)
8.3 Give the value of the following angles:
8.3.1 The angle of incidence on the face AB (1)
8.3.2 The angle of refraction on the face AB (2)
8.4 The diagram below shows an object placed between 2F and F from the lens.
Ray diagrams are used to determine the position and size of images formed.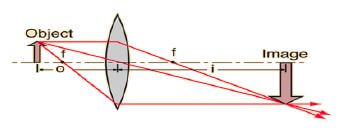
8.4.1 Give THREE characteristics of the image formed in the diagram. (3)
8.4.2 Name TWO practical uses of the above image formation. (2) [12]
QUESTION 9 (Start on a NEW page.)
The diagram below shows the different radiations of the electromagnetic spectrum. They are arranged according to their increasing frequencies.
9.1 Define an electromagnetic wave. (2)
9.2 Give THREE industrial applications of electromagnetic radiation. (3)
9.3 Why are ultraviolet rays considered to be harmful radiations? (2)
9.4 Calculate the energy of a photon of electromagnetic wave with a wavelength of 650 nm. (5)
9.5 Suppose an electromagnetic wave of wavelength 665 nm is available instead. How will the energy of this photon compare with that calculated in QUESTION 9.4? (Write only GREATER THAN, SMALLER THAN or EQUAL TO.) (1) [13]
TOTAL: 150
DATA TABLES FOR TECHNICAL SCIENCE GRADE 12
PAPER 2
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,01 x 105 Pa |
Standard temperature | Tθ | 273 K |
Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT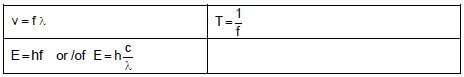
TABLE 3: ELECTROCHEMISTRY
Eθcell = Eθcathode - Eθanode |
TABLE 4A: STANDARD REDUCTION POTENTIALS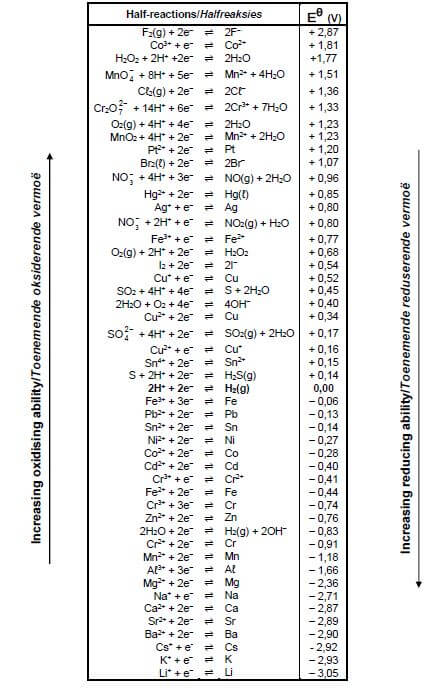
TABLE 4B: STANDARD REDUCTION POTENTIALS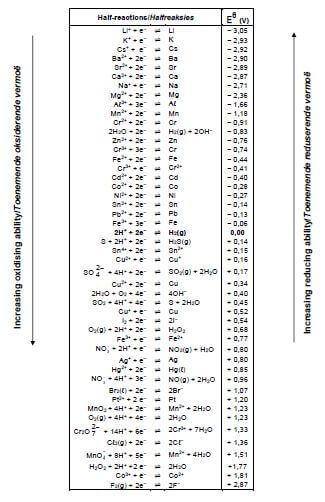
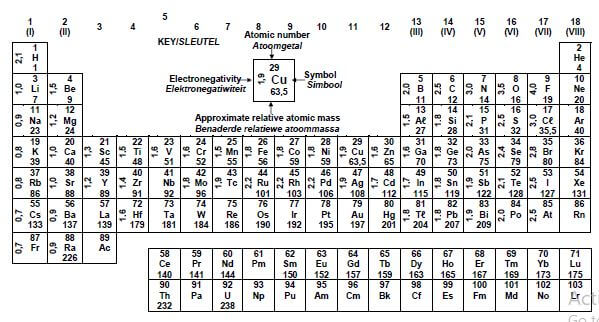
TABLE 3: THE PERIODIC TABLE OF ELEMENTS