TECHNICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS NOVEMBER 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NOVEMBER 2019
NATIONAL SENIOR CERTIFICATE
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of NINE questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You are advised to use the attached DATA SHEETS.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 D.
1.1 Which ONE of the following organic molecules consists of single bonds only?
- Propene
- Propanol
- Propanoic acid
- Propylmethanoate (2)
1.2 Which ONE of the following organic molecules is used as fuels?
- Carboxylic acids
- Aldehydes
- Alkanes
- Esters (2)
1.3 Consider the two structural formulae given below.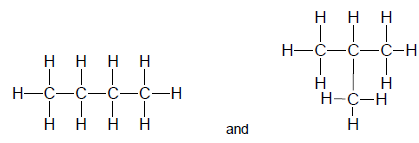
The two structures represent … isomers.
- chain
- positional
- functional
- unsaturated (2)
1.4 Identify the product formed during the addition reaction of but-2-ene.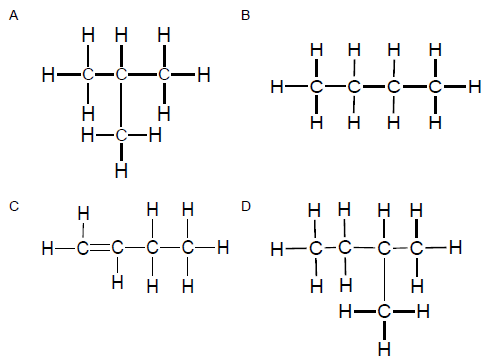
1.5 In which ONE of the following is chemical energy converted to electrical energy?
- Oxidation reaction
- Electrolytic cell
- Galvanic cell
- Electrolysis (2)
1.6 What is the oxidation number of chlorine in CuC2?
- +2
- -2
- +1
- -1 (2)
1.7 Total internal reflection of light is possible if the angle of incidence is …
- equal to the critical angle.
- greater than the critical angle.
- equal to the angle of refraction.
- less than the angle of refraction. (2)
1.8 An object should be placed … to obtain an enlarged and inverted image on a screen when a convex lens is used.
- at F
- at 2F
- between F and 2F
- between the optical centre and F (2)
1.9 Which colour of white light is refracted the most during dispersion?
- Red
- Blue
- Green
- Violet (2)
1.10 In the diagram below, a learner traced the path of a ray of light through a glass slab for DIFFERENT VALUES of the angle of incidence (?) and measured the corresponding values of the angle of refraction (?) and the angle of emergence (?).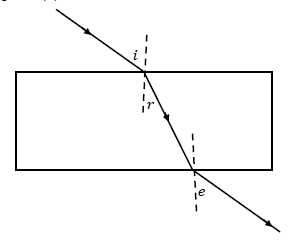
What will be the relationship between ?, ? and ??
- ? > ? > ?
- ? = ? > ?
- ? < ? < ?
- ? = ? < ? (2)
[20]
QUESTION 2 (Start on a new page.)
Organic molecules are used in a variety of industries including food, pharmaceuticals, fuels and construction. They include homologous series such as alkanes, alkenes, alkynes, haloalkanes, alcohols, carboxylic acids, ketones, aldehydes and esters.
2.1 Define the term homologous series. (2)
2.2 Identify any ONE homologous series that is a hydrocarbon in the list above. (1)
2.3 Hydrocarbons can either be saturated or unsaturated. Distinguish between saturated and unsaturated hydrocarbons. (2)
The table below represents organic compounds with different functional groups.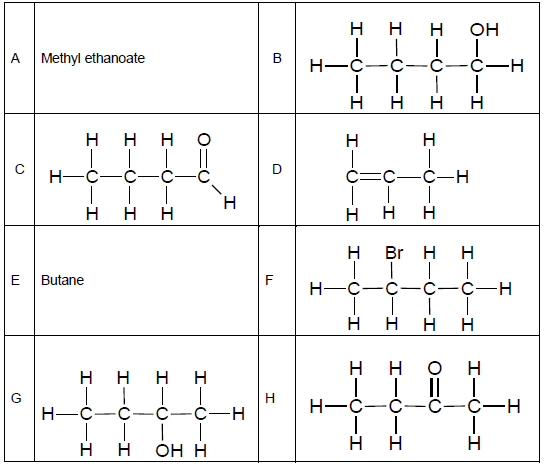
2.4 Define the term functional group. (2)
2.5 Refer to the table of organic compounds on the previous page.
Write down the letter/letters that represent(s) the following:
2.5.1 Haloalkane (1)
2.5.2 Ester (1)
2.5.3 Molecule with a general formula, CnH2n (1)
2.5.4 TWO pairs of organic molecules that are isomers (2)
2.6 Rewrite the identified pairs of isomers in QUESTION 2.5.4 and next to each, write down the TYPE of isomer to which each pair belongs. (2)
2.7 Write down the structural formulae of:
2.7.1 Compound E (2)
2.7.2 A functional group of compound A (2)
2.8 Write down the IUPAC names of the compounds represented by the following letters:
2.8.1 F (2)
2.8.2 H (2)
2.9 Polymers are organic molecules used in everyday life.
2.9.1 Define the term polymer. (2)
2.9.2 Identify TWO objects in the pictures below that are made from polymers.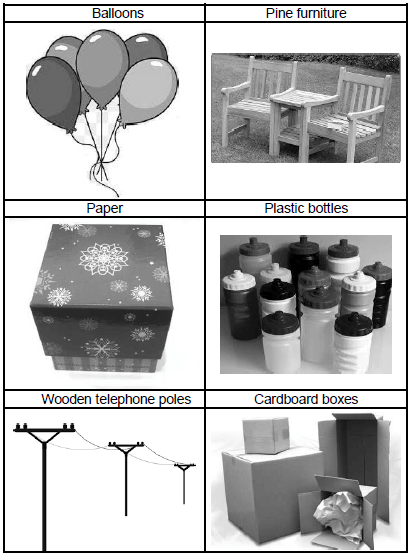
(2)
[26]
QUESTION 3 (Start on a new page.)
The table below shows TWO organic compounds with their corresponding boiling points.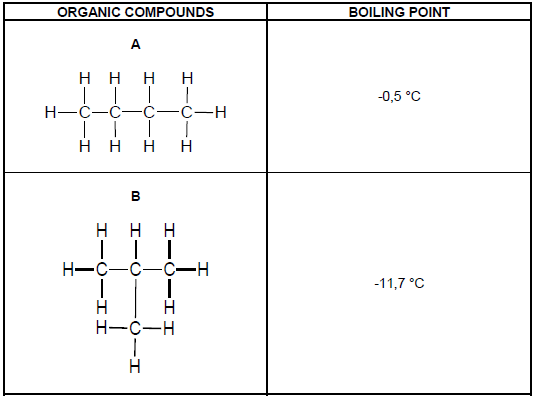
3.1 Identify the TYPE of intermolecular force of the compounds in the table above. (1)
3.2 Explain the difference in the boiling points of A and B. Refer to CHAIN LENGTH, STRENGTH OF INTERMOLECULAR FORCES and ENERGY. (3)
3.3 Define the term melting point. (2)
3.4 Which ONE of the two compounds in the table has a higher melting point? (1)
3.5 Explain the answer in QUESTION 3.4. (2)
3.6 How does the vapour pressure of A compare to the vapour pressure of B?
Write only HIGHER THAN, LOWER THAN or THE SAME AS. (1)
[10]
QUESTION 4 (Start on a new page.)
Butane can react with excess oxygen and is used as cigarette lighter fuel or in bottled gas for cooking.
4.1 Write down the TYPE of reaction that takes place when butane is used as a fuel. (1)
4.2 Consider the flow diagram below and answer the questions that follow.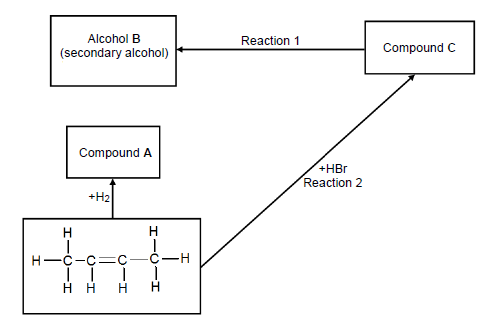
Write down the NAME/TYPE of the following:
4.2.1 Reaction 1 (1)
4.2.2 Reaction 2 (1)
4.3 Write down the NAME of the following:
4.3.1 Compound A (2)
4.3.2 Alcohol B (2)
4.3.3 Compound C (2)
4.4 Name ONE reaction condition for reaction 1. (1)
[10]
QUESTION 5 (Start on a new page.)
The diagram below represents an electrochemical cell used in the decomposition of copper(ll)chloride.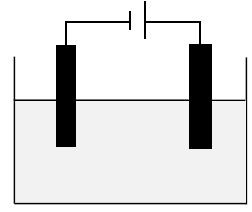
5.1 Identify the TYPE of electrochemical cell represented in the diagram above. (1)
5.2 What energy conversion takes place in this cell? (2)
5.3 Define the term electrolyte. (2)
5.4 What type of ions are Cu2+? Write only ANIONS or CATIONS. (1)
5.5 Write down the balanced half reactions that occur at the:
5.5.1 Cathode (2)
5.5.2 Anode (2)
5.6 Define the term oxidising agent. (2)
5.7 Identify the reducing agent in this cell. (1)
5.8 In which direction will electrons flow in the external circuit? Write only FROM THE CATHODE TO THE ANODE or FROM THE ANODE TO THE CATHODE. (1)
5.9 Write down the overall net cell reaction. (3)
[17]
QUESTION 6 (Start on a new page.)
The picture below is a representation of an electrochemical process operating similarly to a galvanic cell, where an underground iron pipe is connected to the magnesium bar to prevent the iron pipe from rusting.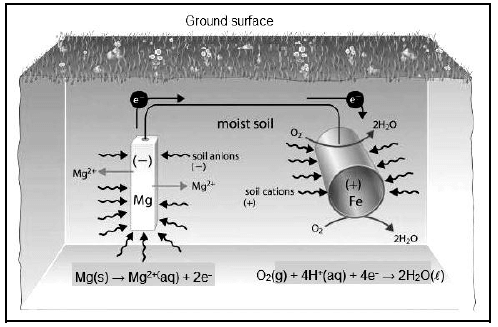
6.1 Define the term galvanic cell. (2)
6.2 Which electrode is the anode? (1)
6.3 What substance in the picture acts as the salt bridge? (1)
6.4 Write down TWO functions of a salt bridge. (2)
6.5 What will happen to the mass of magnesium during the process? Write only INCREASES, DECREASES or STAYS THE SAME. (1)
6.6 Explain the answer to QUESTION 6.5. (2)
6.7 Write down the balanced net reaction. (3)
6.8 Calculate the emf of the cell. (4)
6.9 Eskom is the main supplier of electrical energy in South Africa.
Write down:
6.9.1 THREE alternative sources of energy used in South Africa (3)
6.9.2 THREE advantages of biodiesel (3)
[22]
QUESTION 7 (Start on a new page.)
7.1 State the law of reflection of light. (2)
7.2 How should a ray of light be incident on a rectangular glass block for it to emerge from the opposite side of the block without being refracted? (1)
7.3 An incident light ray, OA, enters a rectangular glass block from air with an incidence angle (i) of 55°. The angle of refraction (r) is 40°. After refraction, the ray of light continues in the glass block and strikes the boundary at B, emerging into air.
Draw a fully labelled ray diagram (not to scale) to show the path of the light ray through the rectangular glass block. Indicate the magnitude of all angles. (6)
7.4 The magnitude of the critical angle in the diagram below is 55°.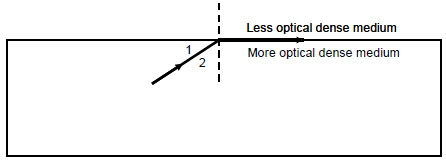
7.4.1 Define the term critical angle. (2)
7.4.2 Which angle represents the critical angle? Write down only 1 or 2. (1)
7.4.3 What will be observed? Write only THE RAY WILL MOVE OUT OF THE MORE OPTICAL DENSE MEDIUM or THE RAY WILL BE REFLECTED BACK INTO THE MORE OPTICAL DENSE MEDIUM. (1)
7.4.4 Write the NAME of the phenomenon observed in QUESTION 7.4.3. (1)
7.4.5 Define the phenomenon in QUESTION 7.4.4. (2)
7.5 Learners performed an experiment to investigate the properties of an image in a flat mirror.
7.5.1 What TYPE of image is formed? (1)
7.5.2 Compare the image and the object with regard to the following:
- Distance in front and behind the mirror (1)
- Size (1)
[19]
QUESTION 8 (Start on a new page.)
8.1 Name the phenomenon where white light is spread into its component colours. (1)
8.2 What attributes of light causes it to spread out into different colours? (2)
8.3 What is the NAME given to a range of colours observed when white light spreads out into its component colours? (1)
8.4 Which photons between YELLOW and GREEN light have higher energy? Write only YELLOW LIGHT or GREEN LIGHT. (1)
8.5 Explain the answer to QUESTION 8.4. (2)
8.6 A girl directs a thin beam of light from different directions onto a convex lens held vertically. At a particular point in the lens the ray of light passes straight through the lens.
Give a reason for this observation. (1)
8.7 What type of lens has a centre that is thinner than the edges? (1)
8.8 Draw a ray diagram to show the POSITION and NATURE of the image formed when the object is placed beyond F of the lens, as described in QUESTION (5)
[14]
QUESTION 9 (Start on a new page.)
9.1 Define a photon. (2)
9.2 Identify the TYPE of electromagnetic wave that is applicable to the function or use depicted in each of the pictures below. Write down the NAME of the electromagnetic wave next to the letter, e.g. H – visible light waves. (6)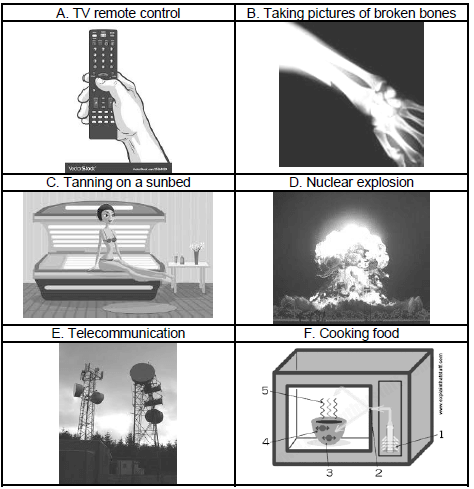
9.3 The wavelength of one of the waves is 600 nm. Calculate the energy of its photon. (4)
[12]
TOTAL: 150
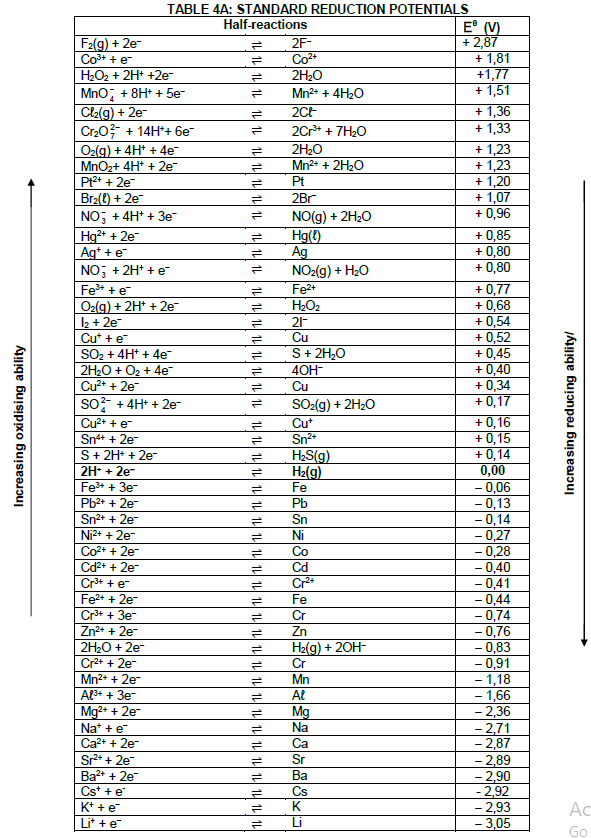
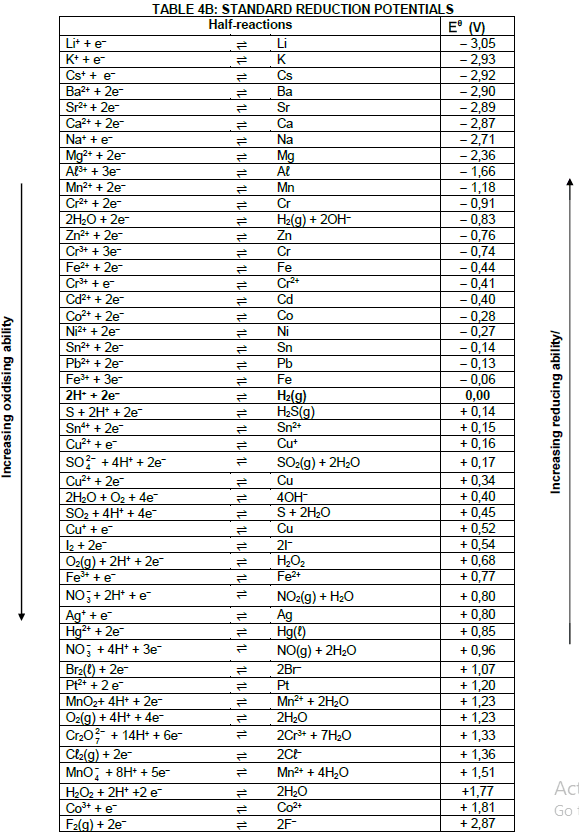
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,01 x 105 Pa |
| Standard temperature | Tθ | 273 K |
| Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
| Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT
| WAVES, SOUND AND LIGHT | |
| v = f λ | T = 1 f |
| Energy | E = hf |
TABLE 3: FORMULAE
| Eθcell= Eθcathode - Eθanode Eθcell = Eθreduction - Eθoxidation Eθcell= Eθoxidising agent - Eθreducing agent |