PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS NOVEMBER 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (P2)
GRADE 12
NOVEMBER 2019
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
QUESTION 1
1.1 D ✓✓ (2)
1.2 C ✓✓ (2)
1.3 B ✓✓ (2)
1.4 D ✓✓ (2)
1.5 C ✓✓ (2)
1.6 B ✓✓ (2)
1.7 B ✓✓ (2)
1.8 A ✓✓ (2)
1.9 A ✓✓ (2)
1.10 C ✓✓ (2)
[20]
QUESTION 2
2.1
2.1.1 CnH2n - 2 ✓ (1)
2.1.2 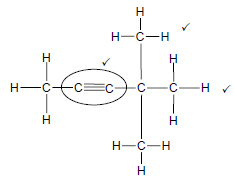
Marking criteria
- Functional group correct. ✓
- 2 methyl substituents. ✓
- Whole structure correct:
(3)
2.2.1 Compounds with the same molecular formula, ✓ but different positions of the side chain/substituents/functional groups ✓on the parent chain. (2)
2.2.3 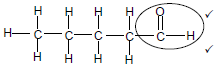
Marking criteria
- Whole structure correct: 2/3
- Only functional group correc Max: ½
OR: Any correct structure of an aldehyde with five carbon atoms. (2)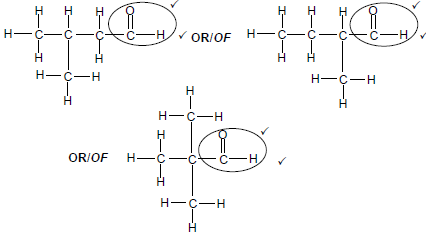
2.2
2.2.2 Pentan-3-one ✓✓
Marking criteria
- Functional group and correct position i.e. ✓
- Whole name correct ✓
2.3
2.3.1 Tertiary (alcohol) ✓
The C atom bonded to the functional group/hydroxyl (group)/-OH is bonded to three other C atoms. /The C-atom bonded to the hydroxyl (group) has no hydrogen atoms. ✓ (2)
2.3.2 2-methylbutan-2-ol
Marking criteria
- 2-methyl ✓
- Butan-2-ol ✓
- Any error e.g. hyphens omitted and/or incorrect sequence: (2)
2.3.3 2-methylbut-2- ene
Marking criteria
- 2-methyl
- But-2-ene/2-butene
- Any error e.g. hyphens omitted and/or incorrect sequence: (2)
[16]
QUESTION 3
3.1 Marking guidelines
The underlined key phrases must be used in the CORRECT CONTEXT (pressure/boiling).
The temperature ✓ at which the vapour pressure of a substance equals atmospheric/external pressure. ✓ (2)
3.2 (Q, R and S) have same molecular mass/formulae/number of carbon and hydrogen atoms/are (chain) isomers. ✓
OR
The compounds are all alkanes /same homologous series and have the same number of carbon atoms. (1)
Marking guidelines
- 55 (°C) ✓
- Compare all three compounds or Q and S in terms of branches/chain lengths / surface area. ✓
- Compare strengths of all three or Q and S’s IMF’
- Compare energy of all three ✓
3.3 55 (°C) ✓
Compare compound R with compounds Q and S:
- Compound R is less branched/compact/spherical/surface area than compound Q and more branched/compact/spherical/surface area than compound S. ✓ OR Q is the most branched/compact /spherical/surface area and S is least branced/compact/spherical/surface area.
- Intermolecular forces in compound R are stronger than in compound Q and weaker than in compound S. ✓
- More energy needed to overcome intermolecular forces in compound R than in compound Q and less energy needed to overcome (break) intermolecular forces in compound R than in compound S. ✓
OR - Compound R has a longer chain length than compound Q and a shorter chain length than compound S.✓ OR S has the longest chain length and Q the shortest.
- Intermolecular forces increase with increase in chain length. ✓
- More energy needed to overcome intermolecular forces as chain length increases. ✓(4)
3.4
3.4.1 P ✓✓ (2)
3.4.2 Marking guidelines
- Name type of IMFs in P/pentanal. ✓
- ame type of IMFs in
- Compare strength of IMFs.
OR Compare energy needed to overcome IMFs. - In P/ pentanal/aldehydes: dipole-dipole forces ✓(in addition to London forces/dispersion forces/induced dipole forces).
- In T/pentan-1-ol: Hydrogen bonding.✓ (in addition to London forces/dispersion forces/induced dipole forces).
- Intermolecular forces in P/pentanal are weaker ✓than in T/pentan-1-ol OR dipole-dipole forces are weaker than hydrogen bonds OR intermolecular forces in T/pentan-1-ol are stronger than in P/pentanal.
OR
More energy needed to overcome/break intermolecular forces in T. (3)
[12]
QUESTION 4
4.1 Haloalkane/alkyl halide ✓ (1)
4.2
4.2.1 Elimination/dehydrohalogenation ✓ (1)
4.2.2 Substitution/hydrolysis ✓ (1)
4.2.3 Esterification/condensation ✓ (1)
4.3
4.3.1
- (Mild) heat/Heating
- Dilute (strong base)/(NaOH/KOH/LiOH)
OR
Add water/H2O/Voeg water/H2O by (2)
4.3.2 Propan-1-ol
Marking criteria
- Correct stem and functional group i.e. propanol✓
- Whole name correct: ✓ (2)
4.4 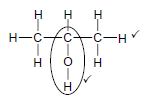
Marking criteria
- Whole structure correct:
- Only functional group correct
Notes
- Accept –OH as condensed.
- Condensed or semi-structural formula:
- Molecular formula:
If functional group is incorrect
If more than one functional group: (2)
4.5 POSITIVE MARKING FROM Q4.3.2 ONLY IF THE COMPOUND IN Q4.3.2 IS AN ALCOHOL.
4.5.1 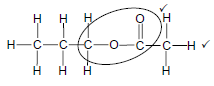
Marking criteria
- Whole structure correct:
- Only functional group correct
Notes
- Condensed or semi-structural formula:
- Molecular formula: 20
- If functional group is incorrect (2)
4.5.2 (Concentrated) sulphuric acid/H2SO4 (1)
[13]
QUESTION 5
5.1 Exothermic
✓H < 0/Energy is released (2)
5.2 rate/tempo =- Δm
Δt
= -0.25 - 2
30
= 0,06 (g·s-1) ✓
(0,0583 g·s-1)
OR
rate/tempo =- Δm
Δt
= - - 1.75
30
= 0,06 (g·s-1) ✓
(0,0583 g·s-1) (3)
Notes
Accept negative answer i.e. - 0,06 g·s-1.
5.3 Marking guidelines
- Calculate: m(CaCO3) reacted of V(CO2) produced. ✓
- Substitute: 100 g∙mol-1. ✓
- USE mol ratio/GEBRUIK molverhouding: n(CO2) : n(CaCO3) = 1 : 1 ✓
- Use of 22,4 dm-3∙mol-1.✓
- Final answer: 0,18 dm3 (0,1792 dm3) ✓
OPTION 1
m(CaCO3) = 40 x 2 ✓
100
= 0,8 g
n(CaCO3)reacted = m/M
= 0.8
100
= 8 x 10-3 mol
n(CO2) = n(CaCO3) ✓
= 8 x 10-3 mol
V(CO2) = 8 x 10-3 x 22,4 ✓
= 0,18 dm3 ✓
OPTION 2
For 2 g antacid
100 g ✓CaCO3 ......22,4 dm3 ✓ CO2
2 g CaCO3 .....0,448 dm3 ✓
100% CO2 .......... 0,448 dm3 ✓
40% CO2 ............. 0,18 dm3 ✓ (5)
OPTION 3
100% CaCO3 .........2 g
40% .......................0,8 g ✓
100 g ✓……. 1 mol
0,8 g .......... 8 x 10-3 mol ✓
1 mol ...............22,4 dm3 ✓
8 x 10-3 mol ......0,18 dm3 ✓
5.4 ANY ONE:
- Concentration (of acid)✓
- Size/mass of tablet/Identical tablet /Type of tablet.
- State of division / Surface area. (1)
5.5 Criteria for conclusion:
- Dependent [(reaction) rate/time] and independent (temperature) variables correctly identified.
- Relationship between the independent and dependent variables correctly stated.
Examples:
- Reaction rate ( 1 ) increases with increase in temperature.
time - Reaction rate ( 1 ) decreases with decrease in temperature.
time - Time taken for reaction decreases when temperature increases.
- Time taken for reaction increases when temperature decreases. (2)
IF
Reaction rate is DIRECTLY proportional to temperature:
5.6
- Increase in temperature increases the average kinetic energy/molecules move faster.
- More molecules have enough/sufficient kinetic energy/More molecules have Ek > Ea. ✓
- More effective collisions per unit time/second. /Frequency of effective collisions increases. ✓ (3)
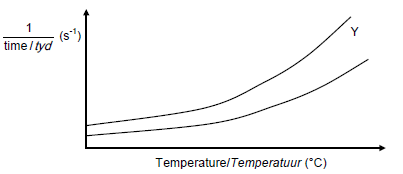
5.7 Marking guidelines
- For each value of temperature, the CURVE Y must be above the given CURVE
- CURVE Y must have an increasing rate with an increase in temperature.(2)
[18]
QUESTION 6
6.1 (The stage in a chemical reaction when the) rate of forward reaction equals the rate of reverse reaction. ✓✓
OR
(The stage in a chemical reaction when the) concentrations of reactants and products remain constant. (2)
6.2 CALCULATIONS USING NUMBER OF MOLES
6.2.1 Marking guidelines
- Substitute: 44 g∙mol-1. ✓
- Equilibrium concentration of CO2 multiply by 3 dm3
- Use mole ratio/Gebruik molverhouding: 1:2 / n(CO) = 2n(CO2). ✓
- n(CO2)change = n(CO2)initial - n(CO2)final
- Correct Kc expression (formulae in square brackets). ✓
- Substitution of concentrations into Kc expression. ✓
- Final answer: 12,24 (range: 11,85 – 12,66) ✓
OPTION 1
n(CO2) = m
M
=60.8
44
= 1,382 mol
| CO2 | CO | |
| Initial quantity (mol) | 1.382 | 0 |
| Change (mol dm3) | 1.22 | 2.44 |
| Quantity at equilibrium (mol) | 0.162 | 2.44 |
| Equilibrium concentration (mol∙dm-3) | 0.054 | 0.813 |
Use ratio
Divide/multiply by 3
KC = [CO]2
[CO]2
=(0.813)2
0.054
= 12.24
No Kc expression, correct substitution
Wrong Kc expression
OPTION 2
n(CO2) = m
M
=60.8
44
= 1,382 mol
n(CO2)change = n(CO2)initial- n(CO2)final
= 1,382 – 0,162
= 1,22 mol
n(CO)change = 2(CO2)
= 2(1,22) ✓
= 2,44 mol
n(CO)eq = n(CO)change = 2,44 mol
c(CO) = n/V
=2.44
3
= 0,813 mol∙dm-3
KC = [CO]2
[CO]2
=(0.813)2
0.054
= 12.24 (Accept range: 11,85 – 12,66).
CALCULATIONS USING CONCENTRATION
Marking guidelines
- Substitute 44 g∙mol-1. ✓
- Initial n(CO2)divide by 3 dm3. ✓
- USE ratio/GEBRUIK verhouding: c(CO2) : c(CO) = 1: 2 ✓✓
- Δc(CO2) = c(CO2)initial/begin - c(CO2) eq/ewe
c(CO)eq/ewe = c(CO)initial/begin + Δc(CO). - Correct Kc expression (formulae in square brackets). ✓
- Substitution of concentrations into Kc expression. ✓
- Final answer: 12,15 (range/gebied: 11,85 – 12,66) ✓
OPTION 3
n(CO2) = m
M
=60.8
44
= 1,382 mol
| CO2 | CO | |
| Initial quantity (mol) | 0.4607 | 0 |
| Change (mol dm3) | 0.4067 | 0.813 |
| Equilibrium concentration (mol∙dm-3) | 0.054 | 0.813 |
KC = [CO]2
[CO]2
=(0.813)2
0.054
= 12.24
No Kc expression, correct substitution
Wrong Kc expression (7)
6.2.2 POSITIVE MARKING FROM Q6.2.1
n(C)reacted = n(CO2)reacted
= 1,22 mol ✓
m(C) = nM
= 1,22(12)
= 14,64 g ✓
Marking guidelines
- USE mol ratio: n(C) = n(CO2). ✓
- Substitute: 12 g∙mol-1. ✓
- Final answer: 14,64 g. ✓ (3)
6.3
6.3.1 Remains the same ✓ (1)
6.3.2 Decreases✓
- (When pressure is increased) the reaction that leads to the smaller amount/number of moles/volume of gas is favoured. ✓
- The reverse reaction is favoured. / More CO2 is formed. ✓ (3)
6.4
6.4.1 Endothermic ✓
- When the temperature increases the mol/percentage CO(g)/product increases/forward reaction is favoured.
- An increase in temperature favours the endothermic reaction (3)
6.4.2 POSITIVE MARKING FROM Q6.2.1.
Marking guidelines
- Calculate total volume/mol of gas at equilibrium: 0,162 + 2,44 = 2,606 dm3 /mol ✓ OR
Calculate the total concentration at equilibrium: 0,054 + 0,813 = 0,867 mol∙dm-3 - Calculate percentage of ANY one gas (CO2 or CO). ✓
- Final answer: T = 827 °C ✓
OPTION 1
Vtotal eq = 0,162 + 2,44 ✓
= 2,606 dm3 (3)
% CO2 =0.162 x 100 ✓
2.606
= 6,225 %
OR
% CO = 2.44 x 100 ✓
2.606
= 93,63 %
OPTION 2
ctotal eq = 0,054 + 0,813
= 0,867 mol∙dm-3
% CO2 =0.054 x 100 ✓
0.867
= 6,228 %
OR
% CO2 =0.813 x 100 ✓
0.867
= 93,77 %
∴ T = 827 °C ✓
[22]
QUESTION 7
7.1 Strong (acid)
Large Ka value > 1 / (HBr) ionises completely (2)
7.2 H2O ✓
Br- ✓ (2)
7.3
7.3.1 Marking guidelines
- Formula: c = n /n = cV / ca × Va = na
V cb × Vb nb - Substitution of: (0,5)(0,0165)/(0,5)(16,5) ✓
- Use mol ratio: 1:1/n(HBr) = n(NaOH) ✓
- Substitute: V = 0,09 dm3 /90 cm3 ✓
- Formula: pH = -log[H3O+] ✓
- Substitute [H3O+] in pH formula. ✓
- Final answer: pH = 1,04 (range: 1,036 – 1,05) ✓
OPTION 1
n(NaOH)reacted/reageer = cV ✓
= 0,5(0,0165) ✓
= 0,00825 mol
n(HBr)excess = n(NaOH) = 0,00825 mol ✓
c(H3O+) = n/V
=0.00825
0.09
= 0,092 mol·dm-3
pH = -log[H3O+] ✓
= -log(0,092) ✓
= 1,04 ✓
OPTION 2
ca Va = na
cb Vb nb
ca(90) = 1
(0.5)(16.5) 1
ca = 0,092 mol·dm-3
pH = -log[H3O+] ✓
= -log(0,092)✓
= 1,04 ✓ (7)
7.3.2 Marking guidelines
- Calculate n(HBr)initial: substitute (0,45)(0,09) in n = cV ✓
- Subtraction:
n(HBr)reacted = n(HBr)initial – n(HBr)reacted with NaOH. ✓✓
OR/OF: c(HBr)reacted = c(HBr)initial – c(H3O+)excess - Use mol ratio: n(Zn(OH)2) : n(HBr) = 1 : 2 ✓
- Substitution of: 99 g∙mol-1 ✓
- Final answer: 1,5964 g (range: 1,58 – 1,68) ✓
POSITIVE MARKING FROM Q7.3.1
OPTION 1
n(HBr)initial = cV
= (0,45)(0,09) ✓
= 0,0405 mol
n(HBr reacted with Zn(OH)2) = 0,0405 – 0,00825 ✓✓
= 0,03224 mol
n(Zn(OH)2) = ½n(HBr) = ½(0,03224) = 0,016125 mol
m(Zn(OH)2) = nM
= (0,016125)(99)✓
= 1,596 g ✓
OPTION 2
c(HBr) = 0,45 – 0,092 ✓✓
= 0,358 mol∙dm-3
n(HBr reacted) = cV
= 0,358 x 0,09 ✓
= 0,0322 mol
n(Zn(OH)2) = ½n(HBr) = ½(0,0322) ✓= 0,01611 mol
m(Zn(OH)2) = nM
= 0,01611 x 99 ✓
= 1,595 g ✓ (1,60 g) (6)
[17]
QUESTION 8
8.1 Chemical to electrical✓ (1)
8.2 Provides path for movement of ions./ Completes the circuit./Ensures electrical neutrality in the cell./Restore charge balance. ✓ (1)
8.3 OPTION 1
Eθcell =Eθcathode - Eθanode
1.49 = 1.36 - Eθanode
Eθanode = 1.36 - 1.49
= -0.13(V)
X is Pb/Lead ✓
Notes
- Accept any other correct formula from the data sheet.
- Any other formula using unconventional abbreviations, e.g. E°cell = E°OA - E°RA followed by correct substitutions:
OPTION 2
Cℓ2 + 2e- → 2Cℓ- Eθ = 1,36 V ✓
X → X2+ + 2e- Eθ = 0,13 V ✓
Cℓ2 + X → X2+ + 2Cℓ- Eθ = 1,49 V ✓
X is Pb/Lead ✓ (5)
POSITIVE MARKING FROM Q8.3
8.4 X/Pb/Lead ✓ (1)
8.5
8.5.1 Reaction reached equilibrium./(In each half cell) the rate of oxidation is equal to rate of reduction./Rate of the forward reaction is equal to the rate of the reverse reaction. ✓ (1)
8.5.2 Increases ✓(1)
8.5.3
- [Cℓ-] decreases. ✓
- Forward reaction is favoured.✓ (2)
[12]
QUESTION 9
9.1 Marking guidelines
If any one of the underlined key phrases in the correct context is omitted, deduct 1 mark.
The chemical process in which electrical energy is converted to chemical energy.✓✓
OR
The use of electrical energy to produce a chemical change.
OR
The process during which an electrical current passes through a solution/molten ionic compound. (2)
9.2
9.2.1 2H2O(ℓ) + 2e- ✓ H2(g) + 2OH-(aq) ✓✓
Ignore phases
Marking guidelines
- H2(g) + 2OH-(aq) ← 2H2O(ℓ) + 2e- (2/2) 2H2O(ℓ) + 2e- ⇌H2(g) + 2OH-(aq) (½)
H2(g) + 2OH-(aq) ⇌ 2H2O(ℓ) + 2e- (0/2) 2H2O(ℓ) + 2e- ← H2(g) + 2OH-(aq) (0/2) - Ignore if charge omitted on electron.
- If charge (-) omitted on OH-
Example: 2H2O(ℓ) + 2e- ✓ H2(g) + 2OH(aq) ✓ Max:½ (2)
9.2.2 Water/ H2O ✓ (1)
9.3 H2O is a stronger oxidising agent ✓ than Na+ ✓and will be reduced ✓ (to H2).
OR
Na+ is a weaker oxidizing agent ✓ than H2O ✓and therefore H2O will be reduced✓ (toH2)
OR
The half-reaction that produces H2(g) has a more positive reduction potential (-0,83 V) ✓ than the half-reaction that produces Na (-2,71 V). ✓
Therefore water/H2O will be reduced ✓ to H2./Na+ will not be reduced to Na. (3)
[8]
QUESTION 10
10.1
10.1.1 Hydrogen/H2 ✓ (1)
10.1.2 Nitrogen monoxide/NO ✓ (1)
10.1.3 Nitric acidr/HNO3 ✓ (1)
10.2
10.2.1 (Catalytic) oxidation/Redox ✓ (1)
10.2.2 NH3 + HNO3 ✓ → NH4NO3 ✓ Bal ✓
Notes
- Reactants ✓ Products ✓ Balancing ✓
- Ignore double arrows (⇌) and phases.
- Marking rule 6.3.10.(3)
10.3
10.3.1 (Total) percentage of nutrients/fertiliser/N,P,K. ✓ (1)
10.3.2 Marking guidelines
- Calculate mass fertiliser in A.
- Calculate mass fertiliser in B.✓
- Calculate mass P in A and B
- Final answer
B has more phosphorous than A. ✓
OPTION 1
Mass fertiliser in A:
Massa kunsmis in A:
m = 21 × 50 = 10,5 kg
100
Mass fertiliser in B:
m = 20 × 40 = 10,8 kg
100
Mass phosphorous in A
3/8 × 10.5 = 3,94 kg
Mass phosphorous in B/
3/8 × 10.8 = 4,05 kg
Fertiliser B has more phosphorous than fertiliser A. ✓
OPTION 2
Mass phosphorous in A
m = 3/8 × 21 × 50= 3,94 kg
100
Mass(P) in B
m = 3/8 × 27 × 40= 4,05 kg
100
Fertiliser B has more phosphorous than fertiliser A.✓
OPTION 3
Mass phosphorous in A
%P = 3/8 × 21= 7,88%
m(P) = 7.88 × 50 = 3,94 kg
100
Mass(P) in B
%(P) = 3/8 × 27 = 10,13%
m =10.13 × 40 = 4,05 kg
100
Fertiliser B has more phosphorous than fertiliser A. (4)✓
OPTION 4
m = 21 × 50 = 10,5 kg
100
Mass fertiliser in B:
m = 20 × 40 = 10,8 kg
100
For the same NPK ratio ✓ the bag with more fertiliser will have more phosphorous ∴ bag B✓
[12]
TOTAL: 150