PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC EXAMS PAST PAPERS AND MEMOS JUNE 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
PAPER 2
GRADE 12
NSC EXAMS
PAST PAPERS AND MEMOS JUNE 2019
MEMORANDUM
QUESTION 1
1.1 B √√ (2)
1.2 B √√ (2)
1.3 C √√ (2)
1.4 A √√ (2)
1.5 D √√ (2)
1.6 C √√ (2)
1.7 A √√ (2)
1.8 D √√ (2)
1.9 A √√ (2)
1.10 D √√ (2) [20]
QUESTION 2
2.1 CONCENTRATED √ (1)
2.2 To prevent reagents escaping √ /To smell the ester/Acts as a condenser (1)
2.3 H2O √ (1)
2.4 propan-1-ol √√ Accept 1-propanol propanol (1/2) (2)
2.5
- n= 54,55/12 √ = 4,55 n = 9,1/1 √= 9,1 n =(100-54.55-9.1) √ /16 √ = 2.27
= 2 = 4- Empirical formula C2H4O √
- Molar mass(R) = 130 + 18 - 60= 88 √
- MMolar Mass (Empirical formula) = 2 x 12 + 4 x 1 + 1 x 16 = 44 √
- Molecular formula = C4H8O2 √ (8) [13]
QUESTION 3
3.1
3.1.1
- Esters √ (1)
-
 (2)
(2)
Marking Criteria
- Whole structure correct2/2
- Only functional group correct ½
3.1.2 Same molecular mass √√ /Same molar mass (2)
3.1.3 B has two sites for hydrogen bonding √. A has one site for hydrogen bonding √ (2)
3.2
3.2.1 Yes √
- Same molecular formula √ but different structural formulae √ (3)
3.2.2 Y √
- D has a larger surface area/chain length than E √
- London forces/Induced-dipole forces √/Dispersion forces stronger√ in D than in E
- More energy needed to break/overcome forces in √
OR
Y √
- E has a smaller surface area/chainlength than D √
- London forces/Induced-dipole forces √/Dispersion forces weaker in E than in D
- Less energy needed to break forces in E √(4)[14]
QUESTION 4
4.1
4.1.1
- Addition √ /Halogenation/Bromination √ (1)
- Elimination √/Dehydrohalogenation √ (1)
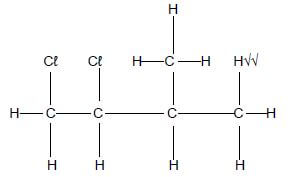
4.1.2
 (3)
(3) - Marking criteria
- Whole structure correct 3/3
- Two Br atoms in structure 1/3
- Marking criteria
- Chlorine √ (1)
4.1.3
 (4)
(4) - Marking criteria
- Whole structure correct 2/2
- Only functional group correct ½
- Marking criteria
- Strong heat √ OR Concentrated strong base (1)
4.2
4.2.1 ADDITION √ (1)
4.2.2 n = 1000 √ (1)
4.2.3 Monomer √ (1)
4.2.4 Make plastics√/ (Any other correct answer) (1)
4.3
4.3.1 Breaking down of a long chain √ /hydrocarbon/alkane into more useful shorter chains √ (2)
4.3.2 THERMAL CRACKING √
TERMIESE KRAKING (1)
4.3.3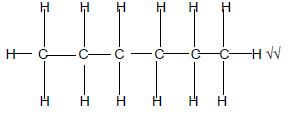 Hexane √√ (4)
Hexane √√ (4)
Marking criteria
- Whole structure correct 2/2
- If one or more hydrogens are omitted ½[22]
QUESTION 5
5.1 Temperature√/Concentration √ (of H2O2)/Add a catalyst √(3)
5.2 Change in concentration per unit time/Rate of change of concentration √√ OR change in amount/volume/mass of reactant/product per unit time. (2)
5.3
5.3.1 Average rate
- = - ∆c/∆t= - (1,45-1,9) √ /(15-0) √
= 0,03√ (mol·dm-3·min-1) (3)
5.3.2 High concentration √√ ( of H2O2 initially)(2)
5.4
5.4.1 ENDOTHERMIC √ (1)
5.4.2 Catalyst increases rate of reaction√
- By lowering activation energy /Deur aktiveringsenergie te verlaag √
- More particles have sufficient Ek to react √/More particles have Ek greater or equal to Ea (4)
5.5
5.5.1 Experiment 1 √ : More particles have higher Ek √(2)
5.5.2 EQUAL TO √Same amount of H2O2 used in both experiments √ (2)
5.6
- nO2 = V/Vm = 0,2/24,8 √ = 8,065 x 10-3 mol
nH2O2 = 2 √ x 8,065 x 10-3
= 0,061 mol
m H2O2 = nM √= 0,0161 x (2+32) √
= 0,547g√ (0,55 g ) (5)
5.7
5.7.1 Q √ (1)
5.7.2 R √ (1)
5.7.3 P √ (1) [27]
QUESTION 6
6.1 Stage reached by a chemical reaction where the rate of forward reaction equals the rate of reverse reaction √√ (2)
6.2 6.2.1 REMAINS THE SAME √ (1)
6.2.2 INCREASES √ (1)
6.2.3 INCREASES √ (1)
6.3 Decreasing pressure is opposed √Reaction which produces more gas moles is favoured √ Forward reaction is favoured √ (3)
6.4 6.4.1 REVERSE √(1)
6.4.2 Catalyst √√ (added)(2)
OPTION 1
6.5 CALCULATIONS USING NUMBER OF MOLES
- Divide by 44 in n = m/M √
- Divide nCO2 equilm & nCO equilm by 2 √
- ∆n(CO2) = ninitial -neq √
- Ratio CO2 : CO 1 : 2 √
- nequil CO = nCOinitial+ ∆n(CO) √
- Kc expression √
- Substituting cequilmCO en cequilimCO2 √
- Final answer √
OPTION 1
ninitial CO2= m/M = 104,72/44 √ = 2,38 mol
| CO2 | C | CO | |
| ninitial | 2,38 | 0 | |
| ∆n | 0,48 | 0,95 (Ratio) | |
| nequilm | 1,9 | 0,96 | |
| 7,6 | 3,84√(Division by 0,25) |
- Kc = [CO]2/[CO2] √
= 3,842/7,6 √
= 1,94 √
OPTION 2
CALCULATIONS USING CONCENTRATION
- Substitute into C=m/MV
- Substitute into c=n/V√
- ∆c(CO2) = cinitial -ceq √
- Ratio CO2 : CO 1:2 √
- cequil CO = cCOinitial+ ∆c(CO) √
- Kc expression√
- Substituting cequilmCO and cequilimCO2 √
- Final answer √
| Initial Concentration of CO2 | Equilibrium concentration of CO2 |
| c= m MV C = 104,72 (44)(0,25) c = 9,52mol.dm-3 | c = n v c = 1,9 0,25 c=3,84mol.dm-3 |
| CO2 | C | CO | |
| Cinitial | 9,52 | - | 0 |
| ∆c | 1,90√ | - | 3,84√(Ratio) |
| c equilm | 7,60 | - | 3,84√ |
- Kc = [CO]2/[CO2] √
= 3,842/7,6 √
= 1,94 √ (8)
6.6
6.6.1 Low √ Yield/
Kc is low √/ Kc is less than 1/ [REACTANTS] > [PRODUCTS]2)
6.6.2 Exothermic √
- As temperature decreases, Kc decreases, [Products]decreases √OR [Reactants increases]
- Reverse reaction is favoured √
- Decrease in temperature favours exothermic reaction √ (4) [25]
QUESTION 7
7.1
7.1.1 Reaction of a salt with water √√ (2)
7.2
7.1.2 Can act as acid and as a base √√/Can donate H+ and accept H+(2)
7.1.3 HCO3- √ (1)
7.1.4 H2CO3 √ (1)
7.2 7.2.1 Standard √ (solution) (1)
7.2.2 CH3COOH √√ (2)
7.2.3
- c1V1=c2V2 n=cV=(0,2)(0.02) √ =0,004 mol
0,2 x 20 √ = 0,16 x V2 √ V=n/c= (0,004)/(0,16) √
V2 = 25 cm3√ OR 0,025 dm3 V=25 cm3 √ OR 0,025 dm3 (3)
7.3 7.3.1 Neutralisation √ (1)
7.3.2 6,8 to 7,2 √ Titration between strong base and a strong acid √ Solution at endpoint is neutral √ (3)
TOTAL: 150
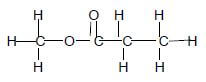 (2)
(2) (3)
(3) 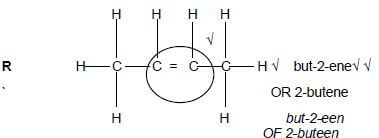 (4)
(4)