PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS MAY/JUNE 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (PAPER 2)
GRADE 12
NATIONAL SENIOR CERTIFICATE EXAMINATIONS
MEMORANDUM
MAY/JUNE 2019
QUESTION 1/VRAAG 1
1.1 C ✓✓ (2)
1.2 A ✓✓ (2)
1.3 C ✓✓ (2)
1.4 A ✓✓ (2)
1.5 D ✓✓ (2)
1.6 C ✓✓ (2)
1.7 D ✓✓ (2)
1.8 D ✓✓ (2)
1.9 C ✓✓ (2)
1.10 A ✓✓ (2)
[20]
QUESTION 2
2.1 Unsaturated ✓
ANY ONE
- C/It has a triple/multiple bond. ✓
- C/It has a triple/multiple bond between C atoms.
- C/It does NOT contain the maximum number of H atoms bonded to C atoms.
- Compound C is an alkyne. (2)
2.2
2.2.1 D ✓ (1)
2.2.2 B ✓ (1)
2.2.3 C ✓ (1)
2.2.4 E ✓ (1)
2.3
2.3.1 ![]() (1)
(1)
2.3.2 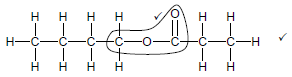 (2)
(2)
Marking criteria:
- Whole structure correct:2/2
- Only functional group correct:½
IF
- More than one functional group: 0/2
- If condensed or semi structural formula used: Max.½
2.3.3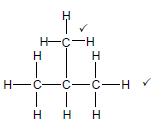
Marking criteria:
- Three C atoms in longest chain. ✓
- One methyl subrstituent on C2
IF
Any error e.g. omission of H atoms, condensed or semi structural formula Max: ½ (2)
2.4
2.4.1
2,3-dibromo-5-methylheptane
Marking criteria:
- Correct stem i.e. heptane. ✓
- All substituents (bromo and methyl) correctly identified. ✓
- IUPAC name completely correct including numbering, sequence, hyphens and commas. ✓(3)
2.4.2 2C4H10 + 13O2 ✓ → 8CO2 + 10H2O ✓ Bal ✓
Notes:
- Reactants ✓ Products ✓ Balancing ✓
- Ignore double arrows and phases.
- Marking rule 6.3.10.
- If condensed structural formulae used: Max.2/3
- Accept coefficients that are multiples.(3)
[17]
QUESTION 3
3.1
3.1.1 Yes✓
ANY ONE:
- Compounds have the same molecular mass. ✓
- Only one independent variable. (2)
3.1.2 Functional group/Homologous series/Type of (organic) compound ✓ (1)
3.2 A/butane ✓
Lowest boiling point/weakest intermolecular forces. ✓(2)
3.3 Marking guidelines
- Type of IMF in A.
- BOTH B and C have hydrogen bonding.
- Compare number of sites for hydrogen bonding in B and C.
- Compare strength of IMFs.
- Compare energy required.
- Between molecules of butane/compound A are London forces/dispersion forces/induced dipole forces. ✓
- Molecules of compound B/propan-1-ol have one site for hydrogen bonding.✓
- Molecules of compound C/ethanoic acid have two/more sites for hydrogen bonding. ✓
- Strength of intermolecular forces increases from compound A/butane to compound B/propan-1-ol to compound C/ethanoic acid. ✓
OR
Intermolecular forces in compound A/butane are the weakest and intermolecular forces in compound C/ethanoic acid are the strongest. - More energy is needed to overcome/break intermolecular forces in compound C than in the other two compounds. ✓(5)
3.4 Butan-1-ol ✓
Longer chain length./Larger molecule./Larger molecular mass./Larger molecular size./Stronger intermolecular forces./Larger surface area.✓(2)
[12]
QUESTION 4
4.1
4.1.1 Addition (polymerisation)✓(1)
4.1.2 Ethene ✓(1)
4.1.3 Polyethene/polythene ✓(1)
4.2
4.2.1 Dehydration/elimination ✓(1)
4.2.2 Catalyst/dehydrating agent/causes dehydration/removes water molecules ✓(1)
4.2.3 Prop-1-ene/propene (2 or 0)(2)
4.2.4 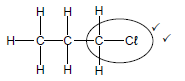
Marking criteria:
- Whole structure correct:
- Only functional group correct: Max: ½
IF:
- More than one functional group: 0/2
- If condensed or semi structural formula used:Max.½ (2)
4.2.5 Addition/Hydration ✓(1)
4.2.6 Propan-2-ol✓✓
Marking criteria:
- Correct stem and functional group i.e propanol ✓
- Name completely correct ✓✓ (2)
[12]
QUESTION 5
5. 1 NOTE
Give the mark for per unit time only if in context of reaction rate.
ANY ONE
- Change in concentration ✓ of products/reactants per (unit) time. ✓
- Change in amount/number of moles/volume/mass of products or reactants per (unit) time.
- Amount/number of moles/volume/mass of products formed/reactants used per (unit) time.
- Rate of change in concentration/amount/number of moles/volume/mass.(2 or/of 0)(2)
5.2.2
| Criteria for conclusion: | |
| Dependent (reaction rate) and independent (concentration ) variables correctly identified. | ✓ |
| Relationship between the independent and dependent variables correctly stated. | ✓ |
Example:
Reaction rate increases with increase in concentration./Reaction rate is proportional to concentration.
IF
DIRECTLY proportional: Max: ½ (2)
5.2
5.2.1 Rate of the reaction ✓ (1)
5.3.2 B ✓ (1)
5.3.3
- At a higher temperature particles move faster/have a higher kinetic energy. ✓
- More molecules have enough/sufficient (kinetic) energy. ✓
- OR
More molecules have (kinetic) energy equal to or greater than activation energy. - More effective collisions per unit time/second./Increased frequency of effective collisions.
- Reaction rate increases. ✓ (4)
5.3
5.3.1 Activation energy/(The boundary line for the) molecules with (adequate) kinetic energy to make effective collisions. ✓(1)
5.4 Curve Y/it was obtained for the reaction where a catalyst was added. ✓
OR
Curve X was obtained for the reaction in the absence of a catalyst. (1)
5.5 Marking guidelines
- Any formula: n= m or c= n ✓
M V - Substitute 0,1 dm3 in n = cV ✓
- Use mole ratio:
n(S)expected = ½n(HCℓ)used ✓ - Substitution of 32 g∙mol-1 in n = m ✓
M - SUBSTITUTE in:
n(S) produced x 100/ m/(S) produced x 100
n(S) expected m(S) expected - Final answer: 56,25% to 60% ✓
| OPTION 1 n(HCℓ)used = cV ✓ = 0,2 x 0,1 ✓ = 0,02 mol n(S)expected = ½n(HCℓ)used = ½(0,02) ✓ = 0,01 mol n(S)produced = m M = 3218,0 = 0,0056 mol %yield =n(S)prod x 100 n(S)exp =0.0056 x 100 0.01 = 56,25% ✓ | OPTION 2 n(HCℓ)used = cV✓ = 0,2 x 0,1 ✓ = 0,02 mol n(S)expected= ½n(HCℓ)used = ½(0,02) ✓ = 0,01 mol m(S)expected= nM = (0,01)(32) ✓ = 0,32 g %yield =m(S)prod x 100 m(S)exp =0.18 x 100 0.32 = 56,25% ✓ |
(6)
[18]
QUESTION 6
6.1 Reversible reaction/Both forward and reverse reactions can take place./Products can be converted back to reactants. ✓(1)
6.2 To favour the forward reaction/production of ammonia./To increase the yield of ammonia./Prevent the decomposition of NH3. (1)
6.3 20(%) ✓ (1)
6.4
6.4.1 At 500 °C lower yield of ammonia:
- The (forward) reaction is exothermic./Reverse reaction is endothermic. ✓
- An increase in temperature favours the endothermic reaction. ✓
- The reverse reaction is favoured.✓
OR
At 350 °C higher yield of ammonia: - The (forward) reaction is exothermic./Reverse reaction is endothermic. ✓
- A decrease in temperature favours the exothermic reaction. ✓
- The forward reaction is favoured. ✓(3)
6.4.2 At 350 atm higher yield of ammonia:
- An increase in pressure favours the reaction that produces the lower number of moles/number of molecules/volume of gas. ✓
- The forward reaction is favoured. ✓
OR
At 150 atm lower yield of ammonia: - A decrease in pressure favours the reaction that produces the higher number of moles/number of molecules/volume of gas. ✓
- Reverse reaction is favoured. ✓(2)
6.5
6.5.1 1 mol N2 reacts with 3 mol H2 to produce 2 mol NH3
∴ 2 mol N2 reacts with 6 mol H2 to produce 4 (mol) NH3 ✓✓ (2 or 0)(2)
6.5.2 POSITIVE MARKING FROM QUESTION 6.5.1.
Marking criteria:
- Calculate 35% of 4 mol NH3 (answer from Q6.5.1)✓
- Use mol ratio n(N2) : n(H2) : n(NH3) = 1 : 3 : 2 ✓
- Equilibrium n(N2) = initial n(N2) - Δn(N2)
Equilibrium n(H2) = initial n(H2) - Δn(H2) - Divide by 0,5 dm3. ✓
- Correct Kc expression (formulae in square brackets). ✓
- Substitution of concentrations into correct Kc expression. ✓
- Final answer: 0,002 ✓
Range: 0,00155 to 0,002 (1,55 x 10-3 to 2 x 10-3)
n(NH3) = 35 × 4
100
= 1,4 mol
| N2 | H2 | NH3 | |
| Initial amount (moles) | 6 | 6 | 0 |
| Change in amount (moles) | 0.7 | 2.1 | 1.4 |
| Equilibrium amount (moles) | 5.3 | 3.9 | 1.4 |
| Equilibrium concentration (mol∙dm-3) | 10.6 | 7.8 | 2.8 |
ratio✓
Divide by 0,5 dm3 ✓
Kc = [NH3] 2
[H2]3 [N2] ✓
= (2.8)2
(7.8)3(10.6) ✓
= 0,002 ✓ (7)
Wrong Kc expression: Max.4/7
No Kc expression, correct substitution: Max.6/7
[17]
QUESTION 7
7.1 A base forms hydroxide ions (OH-) in water/aqueous solution. ✓✓
IF:
A base ionises to form hydroxide ions (OH-).✓. Max.½ (2)
7.2 A strong base ionises/dissociates completely ✓ and a weak base ionises/dissociates incompletely. ✓ (2)
7.3 HCO-3(aq) + H2O(ℓ) ✓ ⇌ H2CO3(aq) + OH-(aq) ✓ Bal. ✓
Accept
NaHCO3(aq) + H2O(ℓ) ⇌ H2CO3(aq) + NaOH(aq)
Notes:
- Reactants ✓ Products ✓ Balancing ✓
- Ignore single arrow.
- Marking rule 6.3.10.
- Ignore phases. (3)
7.4
7.4.1 pH = -log[H3O+] ✓
= -log (0,2) ✓
= 0,70 ✓ (0,699) (3)
7.4.2 Titration of a weak base and a strong acid. ✓
OR
The endpoint will be at pH < 7. (1)
7.4.3 Marking guidelines:
- Any formulae: c= n / n= m =/ Ca x Va = na /c = m
V M cb x Vb nb MV - Substitute 0,2 mol∙dm-3 & 20 x 10-3/0,02 dm3 or 20 cm3. ✓
- Use mol ratio n(XHCO3) : n(HCℓ) = 1 : 1 ✓
- Substitute n(XHCO3) or c(XHCO3) AND 0,4 g. ✓
- M(X) = 39 g∙mol-1 ✓
- X = K/potassium. ✓
| OPTION 1 c(HCℓ) = n V ∴ 0,2 = n 20 x 10-3 n(HCℓ) = 4 x 10-3 mol n(XHCO3) = n(HCℓ) ✓ = 4 x 10-3 mol | OPTION 2 Ca x Va = na cb x Vb nb 0.2 x 20= 1 cb x 100 1 cb = 0,04 mol∙dm-3 c(XHCO3)= m MV ∴0,04 = 0.4 M(0.1) M(XHCO3) = 100 g∙mol-1 M(XHCO3)= M(X) + 61 = 100 ∴M(X) = 39 g∙mol-1 ✓ X = K OR potassium | |
| n= m M ∴4 x 10-3 = 0.4 M M = 100 g∙mol-1 M(XHCO3) = M(X) + 61 = 100 ∴M(X) = 39 g∙mol-1 ✓ X = K ✓ OR potassium | 1 mol M(XHCO3) 4 x 10-3 mol 0,4 g M(XHCO3) = 100 g∙mol-1 M(XHCO3) = M(X) + 61 = 100 ∴M(X) = 39 g∙mol-1 ✓ X = K ✓ OR potassium | |
(6)
[17]
QUESTION 8
8.1 It is a conductor of electricity/a solid to connect wires to./Pt is inert or unreactive.✓OR
Cℓ−(aq) and chlorine gas are not solids and cannot be used as an electrode. (1)
8.2
8.2.1 Chemical (energy) to electrical (energy) ✓ (1)
8.2.2 Cℓ2 + 2e- → 2Cℓ− ✓✓
Marking guidelines
- Cℓ2 + 2e- ⇌ 2Cℓ− ½ 2Cℓ− ⇌ Cℓ2 + 2e- 0/2
2Cℓ− ← Cℓ2 + 2e- 2/2 2Cℓ−- → Cℓ2 + 2e- 0/2 - Ignore if charge omitted on electron.
- If charge (-) omitted on Cℓ− (-):
Max:½ Example: Cℓ2 + 2e- → 2Cℓ ✓ (2)
8.2.3 Cr(s) | Cr3+(aq) ✓|| Cℓ2(g) | Cℓ− (aq) | Pt(s) ✓
OR
Cr(s) | Cr3+(1 mol∙dm-3) || Cℓ2(g) | Cℓ− (1 mol∙dm-3) | Pt(s)
Accept:
Cr | Cr3+ || Cℓ2 | Cℓ− | Pt (3)
8.3
| OPTION 1 Eθcell = Eθreduction = Eθoxidation = 1.36 -(-0.74) Eθcell = 2.10V | Notes
|
| OPTION 2 Cℓ2 + 2e- → 2Cℓ− Eθ = 1,36 V ✓ Cr(s) ✓ Cr3+(aq) + 3e- Eθ = +0,74 V ✓ 2Cr(s) + 3Cℓ2(g) → 2Cr3+(aq) + 6Cℓ−(aq) Eθ = +2,10 V ✓ (4) 8.4 Increases ✓✓ (2) | |
[13]
QUESTION 9
9.1 Electrolytic ✓ (1)
9.2 2H2O + 2e- → H2 + 2OH- ✓✓
Marking guidelines
- 2H2O + 2e- ⇌ H2 + 2OH- ½ H2 + 2OH- ⇌ 2H2O + 2e- 0/2
H2 + 2OH- ← 2H2O + 2e- 2/2 H22 + 2OH- → 2H2O + 2e- 0/2 - Ignore if charge omitted on electron.
- If charge (-) omitted on OH-: Max: 21 Example: 2H2O + 2e- → H2 + 2OH ✓ (2)
9.3
9.3.1 Chlorine (gas) / Cℓ2✓ (1)
9.3.2 P ✓ & Y ✓ (2)
9.4 Cathode✓
Reduction takes place here./Gains electrons.✓ (2)
9.5 CuCℓ2(aq) ✓ → Cu(s) + Cℓ2(g)✓ Bal✓
OR
Cu2+(aq) + 2Cℓ- → Cu(s) + Cℓ2(g)
Notes:
- Reactants ✓ Products ✓Balancing ✓
- Ignore double arrows.
- Marking rule 6.3.10.
- Ignore phases (3)
[11]
QUESTION 10
10.1
10.1.1 II – IV – III - I ✓ (1)
10.1.2 2NH3 + H2SO4 ✓ → (NH4)2SO4 ✓ Bal ✓
Notes:
- Reactants ✓ Products ✓ Balancing ✓
- Ignore double arrows.
- Marking rule 6.3.10. (3)
10.1.3 Vanadium pentoxide (1)
10.1.4 SO3(g) + H2SO4 ✓→ H2S2O7 ✓ Bal ✓
Notes:
- Reactants ✓ Products ✓ Balancing ✓
- Ignore double arrows.
- Marking rule 6.3.10. (3)
10.1.5 Sulphuric acid will form (white) mists./The reaction is very exothermic/gives off too much heat./Corrosive reaction. ✓ (1)
10.2
Marking criteria:
| |
| OPTION 1 m(fertiliser) = 20 × 50 100 = 10 kg m(K) = 2 × 10 X + 3 ∴3,33 ✓ = 2 × 10 X + 3 ∴ X = 3 ✓ | OPTION 3 (fertiliser) = 20 × 50 100 = 10 kg m(P) = ½m(K) ✓ = ½(3,33) = 1,665 kg m(X) = 10 – 3,33 ✓– 1,665 = 5,005 N : P : K = 5,005 : 1,665 : 3,33 = 3 : 1 : 2 ∴ X = 3 ✓ (4) |
| OPTION 2 m(K) = 2 × 20 x 50= 3.33 X + 3 100 X = 3 ✓ | |
[13]
TOTAL: 150