ELECTROCHEMISTRY QUESTIONS AND ANSWERS GRADE 12
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupActivity 1
Give one word for the following statements:
- The chemical process when an electric current is passed through an ionic compound in solution or in molten state. (1)
- An ionic solution that conducts electricity. (1)
- The reactant that donates electrons during a redox reaction. (1)
- The electrode in an electrochemical cell where reduction takes place. (1)
- TRUE OR FALSE? The reactions C(s) + O2(g) → CO2(g) and 2KCℓO3(s) → 2KCℓ(s) + 3O2(g) are examples of redox reactions. (1)
- The electrode in an electrochemical cell where oxidation occurs. (1)
- A substance that shows a decrease in oxidation number during chemical reactions. (1)
- Which of the following substances can be used as an electrolyte?
- Mercury
- Molten copper
- Sugar dissolved in distilled water
- D. Table salt dissolved in distilled water. (1) [8]
Solutions
1. Electrolysis✓ (1)
2. Electrolyte✓ (1)
3. Reducing agent✓ (1)
4. Cathode✓ (1)
5. TRUE statement✓ (1)
6. Anode✓ (1)
7. Oxidising agent✓ (1)
8. D✓ (1) [8]
Activity 2
Give ONE word for the following statements:
- The electrode in a galvanic cell at which reduction occurs. (1)
- Anode in an electrolytic cell. (1)
- The type of electrochemical cell in which electrical energy is converted to chemical energy. (1)
- TRUE or FALSE? An electrolytic cell converts mechanical energy to electrical energy. (1)
- Which ONE of the following half-reactions occurs at the cathode during the electrolysis of an aqueous CuCℓ2 solution?
- Cℓ2 + 2e– → 2Cℓ–
- Cu+ + e– → Cu
- 2Cℓ– → Cℓ2 + 2e–
- Cu2+ + 2e– → Cu (2)
- The gain of electrons by a substance in a chemical reaction is known as …
- Oxidation
- Reduction
- Electrolysis
- D. Oxidation and reduction (2) [8]
Solutions
- Cathode✓ (1)
- Positive electrode✓ (1)
- Electrolytic✓ (1)
- This statement is FALSE. This cell converts electrical energy to chemical energy.✓ (1)
- D ✓✓ (2)
- B ✓✓ (2) [8]
Activity 3
- TRUE or FALSE? During electroplating of a steel teaspoon with silver, the teaspoon is the cathode and the electrolyte is a solution of any soluble compound [2]
Solution
- FALSE. ✓… the electrolyte is a solution of a soluble silver compound. ✓ [2]
b) Refining of copper
Copper which is mined is impure and the copper ore can be refined as follows by means of electrolysis:
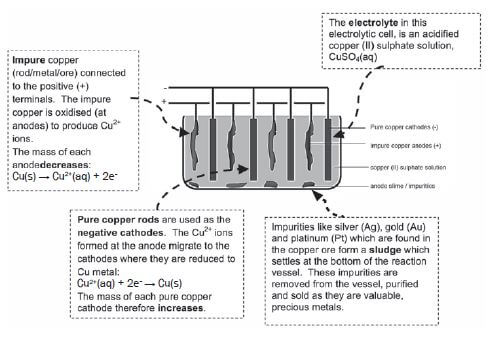
e.g. Worked example
Impure copper can be purified by the process of electrolysis. The simplified diagram represents an electrolytic cell used to purify copper.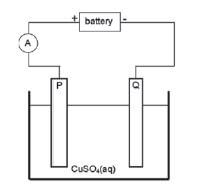
- Define the term electrolysis.
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer.
- Write down the half-reactions that take place at electrodes P and Q.
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process.
- Explain why the concentration of the copper (II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum.
- Why is the sludge of economic importance?
Solutions
- Electrolysis is a process during which electrical energy is converted to chemical energy. It is the process in which electricity is used to bring about a chemical change / decompose / break compounds into components.
- P: P is the positive electrode / anode. The impure Cu is oxidised at the positive electrode / anode.
- P: Anode: Cu(s) → Cu2+(aq) + 2e– oxidation impure
Q:Cathode: Cu2+(aq) + 2e– → Cu(s) reduction pure - Platinum and silver are both weaker reducing agents than copper and will not be oxidised to form ions.
- The rate at which copper is oxidised (at the anode) is equal to the rate at which copper ions are reduced (at the cathode).
- Silver and platinum are valuable and expensive metals and can therefore be sold at a profit.
Activity 4
- Refer to the diagram in the worked example above. One of the electrodes consists of impure copper and the other one of pure copper.
- What type of power source is used to drive the reaction in this cell? Write down only AC or DC. (1)
- Give a reason why the copper(II) sulphate is dissolved in water before it is used in this cell. (1)
When an electric current passes through the solution, electrode P becomes coated with copper. - Is electrode P the cathode or the anode? Support your answer by writing the half-reaction that takes place at electrode P. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
It is found that the impure copper plate contains platinum. The platinum forms a residue at the bottom of the container during electrolysis. - Refer to the relative strengths of reducing agents to explain why platinum forms a residue at the bottom of the container. (2)
- How will the concentration of the copper(II) sulphate solution change during electrolysis? Write down only INCREASES, DECREASES or REMAINS THE SAME. (3) [11]
Solutions
- DC ✓ (1)
- Free ions needed to conduct electricity ✓ (1)
- Cathode. ✓ Cu2+ + 2e– → Cu ✓ (2)
- Cu → Cu2+ + 2e– ✓✓(2)
- Pt is a weaker reducing agent than Cu ✓and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent than Pt and will be oxidised - Remains the same.✓ The rate at which Cu is oxidised at the anode ✓ equals the rate at which Cu2+(aq) is reduced at the cathode ✓ (3) [11]
Activity 5
Give ONE word for the following phrase:
- The main ore from which aluminium is extracted. (1)
- The name of the chemical substance in which Aℓ2O3 is dissolved to lower its melting point during the industrial extraction of aluminium. (1) [2]
Solutions
- Bauxite ✓
- Cryolite ✓ [2]
Activity 6
- In an aluminium smelter, aluminium metal is extracted from bauxite, a hydrated aluminium oxide, via an electrolytic process.
1.1 Write down the energy conversion that takes place in an electrolytic cell. (2)
1.2 Write down the equation for the half reaction responsible for the formation of aluminium metal in a smelter. (2)
1.3 Explain in terms of the relative strength of oxidizing agents why the electrolytic production of aluminum requires more electrical energy than that of iron or copper. (2)
1.4 Name TWO advantages that the use of aluminium has over that of iron. (2) - A huge aluminium smelter is planned for Coega in the Eastern Cape. When operational, it will consume 1350 MW of electricity, or 4% of the nation’s total electrical energy. It is estimated that 5200 jobs will be created at the peak of construction. About 1000 workers will be employed on a full-time permanent basis, and between 200 and 300 full-time subcontractors will also be directly associated with the smelter. (Source: www.engineeringnews.co.za; www.groundwork.org.za).
2.1 Taking the present South African socio-economic realities into account, give ONE reason why the aluminium smelter should:- Not be built (1)
- Be built (1)
2.2 Give ONE reason why environmental activists oppose the construction of the smelter. (1) [11]
Solutions
1.1 Electrical energy → chemical energy ✓✓ (2)
1.2 Aℓ3+ + 3e– → Aℓ ✓✓ (2)
1.3 Aluminium has a lower reduction potential (–1,66 V) ✓/ Weaker oxidizing agent compared to that of iron (–0,44 V) [and copper (+0,34 V)]. The aluminium ions therefore require a large amount of energy to be reduced/ will reduce more difficultly than iron (and copper). ✓ (2)
1.4 It is much lighter for the same strength (or stronger for the same mass). ✓ It is corrosion free.✓ (2)
2.1
- It will consume huge amount of electricity. ✓/ (1) Will cause power failures ✓ (any 2)
- It will create jobs ✓/Create foreign investment. ✓ (1) Contribute to GDP ✓ (any 3)
2.2 The production of the large amount of electricity used ✓ enhances the greenhouse effect (or climate change) ✓ OR The process is responsible for toxic fluoride waste OR pollution. (any one) (1) [11]
Activity 7
Give ONE word for the following phrase:
- The electrode in a galvanic cell at which reduction occurs. (1)
- The component of a galvanic cell that allows for the movement of ions between the half-cells. (1)
- Which statement is CORRECT for a Zn-Cu galvanic cell that operates under standard conditions? (Standard Conditions are defined in the tables at the beginning of this book)
- The concentration of the Zn2+ ions in the zinc half-cell gradually decreases.
- The concentration of the Cu2+ ions in the copper half-cell gradually increases.
- Negative ions migrate from the zinc half-cell to the copper half-cell.
- The intensity of the colour of the electrolyte in the copper half-cell gradually decreases. (2)
- The reactions below occur in two different electrochemical cells X and Y.
Cell X: CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Cell Y: Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Which ONE of the following correctly describes the substance that forms at the CATHODE of each of these cells?
Cell X | Cell Y | |
| A | Cℓ2(g) | Cu(s) |
| B | Cu(s) | Cu(s) |
| C | Cℓ2(g) | ZnSO4(aq) |
| D | Cu(s) | ZnSO4(aq) |
5. Which one of the following statements regarding a copper-silver galvanic cell is TRUE?
- Silver is formed at the anode
- Copper is formed at the anode
- Silver is formed at the cathode
- Copper is formed at the cathode (2) [8]
Solutions
1. Cathode ✓ (1)
2. Salt bridge ✓ (1)
3. D ✓✓ (2)
4. B ✓✓ (2)
5. C ✓✓ (2) [8]
Activity 8
- Which one of the following solutions can be stored in an aluminium container?
(Use the Table of Standard Reduction Potentials.)- CuSO4(aq)
- ZnSO4(aq)
- NaCℓ(aq)
- Pb(NO3)2(aq) (2) [2]
Solution
1. C ✓✓[2]
Activity 9
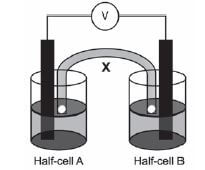
The galvanic cell represented in the diagram consists of a Mg electrode dipped into a Mg(NO3)2 solution, and a Pb electrode dipped into a Pb(NO3)2 solution. Assume that the cell operates under standard conditions.
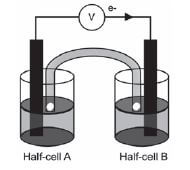
1. State TWO standard conditions under which this cell operates. (2)
2. Write down the half-reaction that takes place in half-cell A. (2)
3. Write down the cell notation for this cell. (2)
4. Calculate the emf of this cell. (3)
5. How will each of the following changes influence the value of the cell’s emf calculated in QUESTION 2.4? Write down only INCREASES, DECREASES or REMAINS THE SAME
5.1 An increase in [Mg2+(aq)] (1)
5.2 An increase in [Pb2+(aq)] (1)
6. In which direction, from half-cell A to B or from half-cell B to A, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer. (3) [14]
Solutions
1. Temperature: 298 K (25 °C) ✓✓(2)
2. Mg(s) → Mg2+(aq) +2e– ✓✓ (2)
3. Mg(s)/Mg2+(1 mol·dm–3) ✓// Pb2+(aq)(1 mol·dm–3)/Pb(s) ✓ (2)
4. E0cell = E0cathode – E0anode = - 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.
5.1 DECREASES ✓ (1)
5.2 INCREASES ✓ (1)
6.
- Half-cell A to half-cell B ✓
- Concentration of positive ions/cations ✓/ Pb2+ ions decreases in half-cell B. ✓
OR - Concentration of positive ions/cations/Mg2+ ions increases in half-cell A
- To prevent a build-up of positive ions in half-cell A and negative ions in half-cell B /for electrical neutrality.
- Positive ions migrate from/through the salt bridge (3) [14]
Activity 10
Learners conduct an investigation to determine which combination of two half-cells will provide the largest emf under standard conditions.
Three half-cells, represented as A, B and C in the table below, are available.
Half-cell A | Half-cell B | Half-cell C |
Mg/Mg2+ | Pb/Pb2+ | Aℓ/Aℓ3+ |
The learners set up galvanic cells using different combinations of the above half-cells.
- Write down the standard conditions under which these cells operate. (2)
- Write down the dependent variable in this investigation. If you can’t remember what the dependent (and independent) variables are, please see the introductory materials at the beginning of this book. (1)
- Use the Table of Standard Reduction Potentials to determine which one of the three half-cells (A, B or C) contains the:
3.1 Strongest reducing agent. (1)
3.2 strogest oxidizing agent. (1) - Without any calculation, write down the combination of two half-cells which will produce the highest emf. Write down only AB, BC or AC. (1)
- One group of learners set up a galvanic cell using half-cells A and B, as shown below. X is one of the components of the galvanic cell.

5.1 Write down the NAME or SYMBOL of the substance that will act as the anode in this cell. Give a reason for the answer. (2)
5.2 Calculate the initial emf of this cell. (3)
5.3 How will an increase in the concentration of the electrolyte in half-cell B affect the initial emf of this cell? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
5.4 Briefly explain how component X ensures electrical neutrality while the cell is functioning. (1) [13]
Solutions
- Temperature 25 °C /298 K ✓
Concentration of electrolytes = 1 mol·dm–3 ✓ (2) - emf/potential difference ✓ (1)
- 3.1 Half-cell A ✓ (1)
3.2 Half-cell B ✓ (1) - Combination AB ✓ (1)
- 5.1 Magnesium/Mg. 3Is oxidised/loses electrons/increase in oxidation number/stronger reducing agent ✓ (2)
5.2 E0cell = E0cathode – E0anode = – 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.3 Increases ✓ (1)
5.4 Allows for the migration of positive ions to the cathode half-cell ✓ (1)
OR
Allows for the migration of negative ions to the anode half-cell [13]
Activity 11
- Which is the the reference electrode in the diagram above? (1)
- TRUE or FALSE? A standard cell that has a negative emf cannot be used as a galvanic cell. (2)
- TRUE or FALSE? The standard conditions used to measure standard electrode potentials are: A temperature of 273 K, a concentration of 1 mol·dm–3, and a pressure of 101,3 kPa. (2)
- Which ONE of the following containers can be used to store an iron(II) sulphate solution?
- Aℓ
- Mg
- Ni
- Zn (2) [7]
Solutions
- Hydrogen half-cell ✓ (1)
- TRUE ✓ (2)
- FALSE. ✓ A temperature of 298 K (25 °C) — the question gives the temperature incorrectly ✓(2)
- C ✓ (2) [7]
Activity 12
Give ONE word for the following phrase:
- The process taking place in a cell when an electric current passing through its electrolyte, results in chemical reactions at its electrodes. (1)
- TRUE or FALSE? A battery labelled as 3 000 mA·h can deliver a current of 500 mA for 6 hours. (2) [3]
Solutions
1. Electrolysis ✓ (1)
2. True. ✓✓ (2) [3]
Activity 13
- The most common filling for tooth cavities is ‘dental amalgam’ – a solid solution of tin and silver in mercury. If you bite on a piece of aluminium foil that is in contact with a dental filling in your mouth, you may feel a painful sensation because
- the aluminium foil is hard.
- a temporary galvanic cell has been set up whilst the aluminium and filling are in contact.
- electrons are being transferred to the aluminium.
- a temporary electrolytic cell has been set up whilst the aluminium and filling are in contact. (2)
- Which one of the following can be classified as a redox reaction?
- NH3(g)+ HNO3(g) →NH4NO3(s)
- FeS(aq)+ 2HCℓ(aq) → FeCℓ2(aq) + H2S(g)
- Aℓ(s) + Cℓ2(g) →AℓCℓ3(s)
- Mg(NO3)2(s) → Mg2+(aq) + 2NO3–(aq) [with H2O catalyst] (2)
- A silver (Ag) spoon is left in a beaker containing copper nitrate, Cu(NO3)2, solution. What will be observed after some time?
- The spoon will be covered with a thin layer of copper.
- The nitrate will form NO2 gas and copper will form in the beaker.
- The spoon will start to erode and its mass will decrease.
- There will be no reaction.
Solutions
1. B. ✓✓ (2)
2. C. ✓✓ (2)
3. D. ✓✓ (2) [6]
Activity 14
- A group of learners set up an electrochemical cell using lead and copper half cells.
1.1 Which ONE of copper or lead will be the negative electrode? Give a reason for your answer. (2)
1.2 Use the Table of Standard Reduction Potentials and write down the reduction half-reaction that will take place in this cell. (2)
1.3 A 2V bulb is connected to the cell. Will the bulb light up? Justify your answer with a calculation. (5)
1.4 A voltmeter is now connected to the cell instead of the bulb. It is observed that after a while the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell. (3) - A cell such as the one described above is not much useful. However, the principle is used in batteries for cars, torches, computers, et cetera. These batteries are called secondary cells. One such battery is the mercury cell. The half reactions occurring in this cell are shown below.
Zn(s) + 2OH–(aq) → ZnO(s) + H2O + 2e–……… (i)
HgO(s) + H2O + 2e– → Hg(ℓ) + 2OH–(aq)….….. (ii)
2.1 Write down the overall cell reaction. (3)
2.2 Why does the use of this battery pose an environmental hazard? (1) [16]
Solutions
1.1 Lead. ✓ Stronger reducing agent ✓ OR Is oxidised preferably (2)
1.2 Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
1.3 E0cell = E0cathode − E0anode ✓ = 0,34– (–0,13) ✓ =0,47V✓ (5) Bulb will not light, ✓ energy from cell not sufficient ✓ OR emf of cell is much less than 2 V needed for the bulb ✓✓
1.4. While the cell is in operation, the concentration of the reactants (Cu2+(aq)) decreases. ✓ At the same time the concentration of the products (Pb2+(aq)) increases. ✓ The result is a gradual decrease in the cell potential until there is no further change in concentration and equilibrium is reached where the cell potential will be zero. ✓ (3)
2.1 Zn(s) + HgO(s) → ZnO(s) + Hg(ℓ) ✓✓✓ (3)
2.2 Mercury is poisonous ✓ (1) [16]
Activity 15
The discovery of electrochemical cells has revolutionised our way of life.
The diagram below represents an electrochemical cell.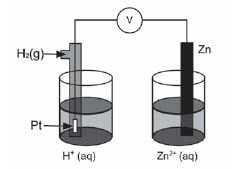
- Name the type of electrochemical cell that converts chemical energy to electrical energy. (1)
- If the electrochemical cell is set up as illustrated, there will be no reading on the voltmeter. Give a reason for this observation.(1)
- Write down the value of the standard emf of the electrochemical cell when it is functioning. (1)
- Write down the voltmeter reading when the net cell reaction in the above electrochemical cell reaches equilibrium. (1)
- Write down the equation for the reaction that occurs at the anode. (1)
- Another electrochemical cell is set up under standard conditions by replacing the standard hydrogen half-cell with a standard magnesium half-cell.
6.1 Which electrode will undergo a decrease in mass? Give a reason for your answer. (2)
6.2 Calculate the initial emf of this electrochemical cell under standard conditions. (3)
6.3 After a while the emf of this electrochemical cell decreases. Explain this observation by referring to the concentration of the electrolytes. (2) - Electrochemical cells such as motor car batteries with plastic casings can harm the environment if not disposed of safely. Suggest TWO ways how motor car batteries can be safely disposed of. (2) [14]
Solutions
1. Galvanic/voltaic cell ✓ (1)
2. Incomplete circuit/No salt bridge ✓ (1)
3. 0,76 V ✓ (1)
4. Zero ✓ (1)
5. Zn(s) → Zn2+(aq) + 2e– ✓ (1)
6.1 Mg. ✓ Mg is oxidised ✓ (2)
6.2 E0cell = E0cathode – E0anode ✓ = 0,76 – (–2,36) ✓ = 1,6 V ✓ (3)
6.3 As the cell functions, the concentration of zinc ions (reactants) decreases ✓ relative to the standard conditions and the concentration of magnesium ions (products) increases relative to standard conditions. The reverse reaction starts opposing the forward reaction causing the emf to decrease relative to standard conditions. ✓ (2)
7. Neutralise acid before disposal. ✓ Recycle plastic casing and lead electrodes ✓ (2) [14]
Activity 16
In 1780, Luigi Galvani discovered that when copper and zinc metal were connected to each other and if each free end touched different parts of the same nerve of a frog leg at the same time, the frog’s leg contracted. He called this “animal electricity”.
- Briefly explain what this “animal electricity” really was. (1)
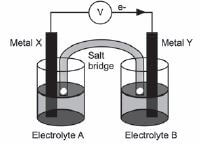
The diagram shows an electrochemical cell setup under standard conditions using aluminium (Aℓ) and nickel (Ni) electrodes. AℓCℓ3(aq) and NiCℓ2(aq) are used as the electrolytes, and a solution of sodium nitrate (NaNO3(aq)) is used in the salt bridge.
Answer each of the following questions on this electrochemical cell:
- The diagram indicates that electrons flow from metal X to metal Y.
Identify:
2.1 Metal X (1)
2.2 Electrolyte B (1) - What is the concentration of electrolyte B? (1)
- Write down the FORMULA of the substance that moves towards metal Y in the salt bridge. (1)
- Write down the half-reaction that occurs at the cathode of this cell. (2)
- Calculate the reading on the voltmeter. (3)
- State what happens to the concentration of metal ions in the solution containing electrolyte as time goes by? (1)
- 8.1 Consider 5.7 again and recall your answer. What effect does this have on the voltmeter reading? (1)
8.2 Briefly explain your answer to 5.8.1. (2) [14]
Solutions
- The chemical reaction between the zinc and the copper r eleased electrical energy ✓ (1)
- 2.1 Metal X: Aℓ ✓ (1)
2.2 Electrolyte B: NiCℓ2 ✓ (1) - 1mol·dm–3 ✓ (1)
- NO3– ✓ (1)
- Ni2++ 2e– → Ni ✓ ✓ (2)
- E0cell = E0oxidising agent − E0reducing agent ✓ = –0,25V–(–1,66V) ✓ =1,41V✓ (3)
- Increase ✓ (1)
- 8.1 The voltmeter reading decreases ✓ (1)
8.2 As the [Aℓ3+] increases, ✓ the reverse reaction in the Aℓ Aℓ3+ half-cell:
Aℓ ⇌ Aℓ3+ +3e– is favoured.✓ (2)
OR
The tendency of the net reaction Aℓ + Ni2+ → Aℓ3+ + Ni to proceed from left to right is reduced. This lowers the electrode potential of this half-cell, resulting in a lower cell potential. [14]
Activity 17
Tina wants to investigate the effect of the area of the metal plates used as electrodes in a galvanic cell on the emf of the cell. She sets up the following Zn/Pb cell under standard conditions and measures the emf.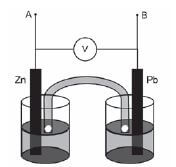
- Which electrode will show an increase in mass when this cell is functioning? (1)
- Write down the equation for the half-reaction occurring at the anode. (2)
- Calculate the emf that you would expect Tina to read on the voltmeter. (3)
- Name TWO variables that should be controlled for during this investigation. (2)
- Tina now replaces the two metal plates with ones of larger surface area, and takes the readings again.
5.1 How would you expect the new emf to compare with the one calculated in Question 6.3? (Only write SMALLER THAN, LARGER THAN or EQUAL TO) (1)
5.2 Explain your answer to Question 5.1 (1) - Tina now connects a resistor of low resistance across terminals A and B. She notes that the reading on the voltmeter immediately drops.
6.1 Give a reason for this observation. (1)
6.2 After some time she observes a further drop in the reading on the voltmeter. Give a reason for this observation. (2) [13]
Solutions
- Pb ✓
- Zn → Zn2+ + 2e– ✓✓
- E0cell = E0oxidising agent − E0reducing agent✓ –0,13 – (–0,76) ✓ =0,63V✓ (3)
- Temperature, ✓ (initial) concentration of electrolytes ✓(2)
- 5.1 Equal to ✓ (1)
5.2 Area/size of electrodes has no effect on the emf of a cell. It is still a standard cell ✓ (1) - 6.1 the cell has internal resistance ✓ (1)
6.2 The emf decreases as the concentration of Pb2+(aq) decreases. ✓/The cell is running flat as the electrolyte concentration in the Pb cell decreases. ✓✓ (any 2) (2) [13]
Activity 18
Electrolysis is an important industrial process used to decompose compounds, extract metals from their ores and to purify metals like gold or copper.
The simplified diagram below represents an electrolytic cell used to purify copper.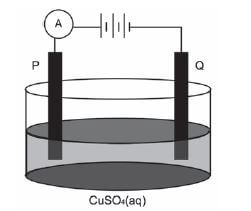
- Define the term electrolysis. (2)
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process. (2)
- Explain why the concentration of the copper(II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum. (2)
- Why is the sludge of economic importance? (1) [11]
Solutions
- The process in which electricity is used to bring about a chemical change / decompose / break compounds into components ✓✓(2)
OR
A process in which electrical energy is converted to chemical energy. - P. ✓
P is the positive electrode /anode ✓ (2)
OR
Oxidation takes place at the positive electrode/anode - Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
- Pt and Ag are both weaker reducing agents ✓ (than copper) and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent (than Pt and Ag) and will be oxidised - The rate at which copper is oxidised at the anode, ✓ is equal to the rate at which copper ions are reduced at the cathode. ✓ (2)
- Contains platinum and silver that are valuable/expensive metals ✓ (1) [11]