ElimuZA Access to Education
THE FERTILISER INDUSTRY QUESTIONS AND ANSWERS GRADE 12
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupActivity 1
- Which of the following is a primary mineral nutrient that is needed by plants?
- N
- C
- Mg
- Na (2) [2]
Solution
1. A [2]
Activity 2
- Which ONE of the following is NOT associated with eutrophication in water?
- Dead zones
- Algal bloom
- Depletion of oxygen
- Increased aquatic life (2) [2]
Solution
- D [2]
Activity 3
Fertilisers allow farmers to grow crops in the same soil year after year. However, environmental problems, such as eutrophication, are associated with the application of fertilisers.
- State ONE PRECAUTION that a maize farmer can take to prevent eutrophication. (1)
Nitric acid is an important reactant in the production of ammonium nitrate, a nitrogen-based fertiliser. - Write down the name of the industrial process for the production of nitric acid. (1)
- Write down a balanced equation for the preparation of ammonium nitrate from nitric acid. (3) [5]
Solutions
- Use fertilisers sparingly / Do not over-fertilise
Make use of precision (computerised) application of fertilisers
Ensure that water from fields does not run into rivers/dams
Redirect water from fields into reservoirs/away from rivers/dams (any one) (1) - Ostwald process (1)
- HNO3 + NH3 → NH4NO3 (3) [5]
Activity 4
The rapidly increasing human population is resulting in an ever-increasing demand for food. To meet this demand, farmers apply fertiliser to the same cultivated land EACH YEAR.
- Explain why farmers have to apply fertilisers to their land each year. (1)
- Write down one negative impact that over-fertilisation can have on humans. (1)
- Sulphuric acid is an important substance used in the manufacture of fertilisers.
The equation below represents one of the steps in the industrial preparation of sulphuric acid.
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H<0
3.1 Write down the name of the process used to prepare sulphuric acid in industry (1)
3.2 Write down the name or the formula if the catalyst used in 2.3.1 (1)
3.3 Is the forward reaction endothermic or exothermic? Give a reason for your answer. (1)
3.4 Write down the name or formula of the fertiliser formed when sulphuric acid reacts with ammonia. (2) [7]
Solutions
- Fertilisers replenish nutrients depleted by the growing of crops (1)
- Damage to crops/soil resulting in small or no harvest/ less income. Excessive fertiliser seeps into groundwater and contaminates drinking water or runs into rivers and/or dams and causes eutrophication which may result in less income /famine/starvation /poor quality drinking water /fewer recreation areas/ environmental damage/ death of wild animals (any one) (1)
- 3.1 Contact process (1)
3.2 V2O5 vanadium pentoxide (any one) (1)
3.3 Exothermic as∆H < 0 (1)
3.4 (NH4)2SO4 OR / Ammonium sulphate (2) [7]
Activity 5
Ammonia, ammonium nitrate and ammonium sulphate are three important nitrogen-containing fertilisers. The flow diagram below shows how these fertilisers are produced in industry.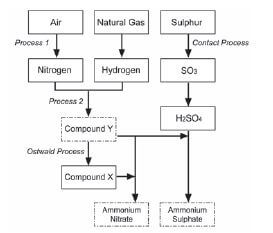
- Use the information in the flow diagram above and write down the following:
1.1 Name Process 1 (1)
1.2 Balanced equation for Process 2 (3)
1.3 Name or Formula for compound X (1)
1.4 Balanced equation for the preparation of ammonium sulphate using sulphuric acid and compound Y (3)
1.5 Name or Symbol of the primary nutrient in ammonium sulphate (1) - Write down one positive impact of fertilisers on humans (1)
- Write down two negative impacts of the use of ammonium nitrate as fertiliser, on humans. (2) [12]
Solutions
- 1.1 Fractional distillation of liquid air (1)
1.2 N2 + 3H2 → 2NH3 (reactants products balance (3)
1.3 Nitric acid / HNO3 (1)
1.4 H2SO4 + 2NH3 → (NH4)2SO4 (reactants products balance (3)
1.5 Nitrogen / N (1) - Enhance growth of crops/plants to produce more food for humans food security for humans production / application of fertiliser results in job creation selling of fertilisers stimulates the economy (any one) (1)
- (Excessive) nitrates in water (eutrophication) can result in blue baby syndrome or cancer (Excessive) nitrates/ammonium ions in water can result in poor quality drinking water or death of fish or less food or fewer recreational facilities or famine due to killing plants / crops from the excess or excessively changing the pH of the soil and thereby reducing the food production (any two) (2) [12]