ELECTROCHEMISTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Summary
- Key concepts: Definitions and terminology
- Electrochemical cells
- Electrolytic cells
- Application of electrolysis
- Voltaic (Galvanic) cells
- Cell notation
- Standard electrode potentials
- The standard hydrogen electrode
- The emf of an electrochemical cell
Electrochemistry
Electrochemistry refers to chemical reactions during which chemical energy is converted to electric energy, or electric energy is converted to chemical energy. During these chemical reactions oxidation and reduction take place. These are called redox reactions.
5.1 Summary
Redox (reduction - oxidation) reactions:
- Electron transfers take place.
- can be represented by two half-reactions:
- an oxidation half-reaction and
- a reduction half-reaction.
5.2 Key concepts: Definitions and terminology
NB
- Electrolysis is the chemical process in which electrical energy is converted to chemical energy.
- Electrolysis is the process where electrical energy is used to produce a chemical change.
Another name for a galvanic cell is a voltaic cell. A galvanic cell has self-sustaining electrode reactions.
Remember
You must remember these basic definitions for electrochemistry:
- Galvanic cells
- Electrolytic cells
- Oxidation
- Reduction
- Oxidising agent
- Reducing agent
- Anode
- Cathode
- Electrolyte
- Electrolysis
A galvanic cell is a cell in which chemical energy is converted into electrical energy.
An electrolytic cell is a cell in which electrical energy is converted into chemical energy.
NB: Remember
We define oxidation and reduction in terms of electron (e–) transfer
- RED CAT: REDuction – CAThode
- ANOX: ANode - OXidation
Oxidation | Reduction |
Oxidation is the loss of electrons by a substance (i.e. by an atom, a molecule or an ion). | Reduction is the gain of electrons by a substance (i.e. by an atom, a molecule or an ion). |
Learn: LEO for Loss of Electrons is Oxidation. | Learn: GER for Gain of Electrons is Reduction. (because gaining electrons is gaining minuses, so reducing) |
A substance that is oxidised (i.e. loses electrons) is called a reducing agent. | A substance that is reduced (i.e. gains electrons) is called an oxidising agent. |
The oxidation number of a compound that is oxidised, increases (becomes less negative, or becomes more positive). | The oxidation number of a compound that is reduced, decreases (becomes more negative, or becomes less positive). |
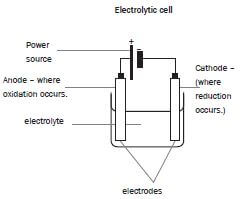
The anode (–) is the electrode where oxidation (+) takes place
The cathode (+) is the electrode where reduction (–) takes place.
The electrodes:
- conduct electricity
- are placed in electrolytes which are solutions consisting of ions.
The electrolyte is the solution/liquid/dissolved substance that conducts electricity through the movement of ions.
Activity 1
Give one word for the following statements:
- The chemical process when an electric current is passed through an ionic compound in solution or in molten state. (1)
- An ionic solution that conducts electricity. (1)
- The reactant that donates electrons during a redox reaction. (1)
- The electrode in an electrochemical cell where reduction takes place. (1)
- TRUE OR FALSE? The reactions C(s) + O2(g) → CO2(g) and 2KCℓO3(s) → 2KCℓ(s) + 3O2(g) are examples of redox reactions. (1)
- The electrode in an electrochemical cell where oxidation occurs. (1)
- A substance that shows a decrease in oxidation number during chemical reactions. (1)
- Which of the following substances can be used as an electrolyte?
- Mercury
- Molten copper
- Sugar dissolved in distilled water
- D. Table salt dissolved in distilled water. (1) [8]
Solutions
1. Electrolysis✓ (1)
2. Electrolyte✓ (1)
3. Reducing agent✓ (1)
4. Cathode✓ (1)
5. TRUE statement✓ (1)
6. Anode✓ (1)
7. Oxidising agent✓ (1)
8. D✓ (1) [8]
5.3 Electrochemical cells
Electrochemical cells allow conversion between electrical and chemical energy.
There are two types of electrochemical cells:
- Galvanic (voltaic) cells
- Electrolytic cells.
 |  |
|
|
Activity 2
Give ONE word for the following statements:
- The electrode in a galvanic cell at which reduction occurs. (1)
- Anode in an electrolytic cell. (1)
- The type of electrochemical cell in which electrical energy is converted to chemical energy. (1)
- TRUE or FALSE? An electrolytic cell converts mechanical energy to electrical energy. (1)
- Which ONE of the following half-reactions occurs at the cathode during the electrolysis of an aqueous CuCℓ2 solution?
- Cℓ2 + 2e– → 2Cℓ–
- Cu+ + e– → Cu
- 2Cℓ– → Cℓ2 + 2e–
- Cu2+ + 2e– → Cu (2)
- The gain of electrons by a substance in a chemical reaction is known as …
- Oxidation
- Reduction
- Electrolysis
- D. Oxidation and reduction (2) [8]
Solutions
- Cathode✓ (1)
- Positive electrode✓ (1)
- Electrolytic✓ (1)
- This statement is FALSE. This cell converts electrical energy to chemical energy.✓ (1)
- D ✓✓ (2)
- B ✓✓ (2) [8]
5.4 Electrolytic cells
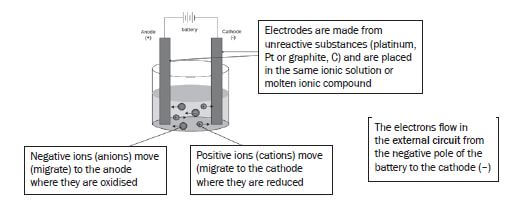
a).Electrolysis of molten ionic compounds:
- The negative ions migrate (move) to the anode where they are oxidised.
- The positive ions migrate to the cathode where they are reduced.
b). Electrolysis of ionic solutions:
The anions (negative ions) from the ionic compound and the hydroxyl ions (OH–) from the water migrate to the anode (positive electrode). The anions from the ionic compound compete with the hydroxide ions (OH–) from the solution, to be oxidised.
Rules:
If the ionic compound contains:
- halide ions (Cℓ–; Br– or I–), the halide ion will be oxidised and NOT the hydroxide ion. The product will therefore be the corresponding halogen.
- sulphate (SO42–) or nitrate (NO3–) ions, the hydroxide ion (OH–) will be oxidised to produce oxygen, O2(g).
The cations (positive ions) from the ionic compound and the H+ ions from the water migrate to the cathode (negative electrode).
The cations from the ionic compound compete with the H+ ions to be reduced.
Rules:
If the ionic compound contains:
- cations of metals with a positive electrode potential, these cations will be reduced to form the corresponding metal e.g. Cu. See the table on electrode potentials provided in introduction to this book.
- cations of metals with a negative electrode potential (e.g. Zn2+), the H+ ions will be reduced to form hydrogen, H2(g). See the table on electrode potentials provided in this book.
NB
Remember to indicate the charge on the ion
e.g. Worked example 1
- Explain how the electrolysis of a copper (II) chloride solution CuCℓ2(aq) takes place.
Solution
- The anions Cℓ– and OH– migrate to the positive anode. The Cℓ– ions are oxidised.
oxidation half-reaction: 2Cℓ–(aq) → Cℓ2(g) + 2e–
The cations (Cu2+ and H+) migrate to the cathode. The Cu2+ ions are reduced.
reduction half-reaction: Cu2+(aq) + 2e– → Cu(s)
The net reaction: Cu2+(aq) + 2Cℓ–(aq) → Cu(s) + Cℓ2(g)
The solution is initially blue due to the presence of the Cu2+ ions, but as they are reduced to Cu(s), the solution turns colourless and red-brown Cu(s) is deposited on the cathode.
e.g. Worked example 2
Predict the products of the electrolysis of a copper (II) sulphate solution CuSO4(aq)
Solution
2.
- At the anode: O2(g)
- At the cathode: Cu(s)
e.g. Worked example 3
Predict the products of the electrolysis of a zinc chloride solution ZnCℓ2(aq)
Solution
3.
- At the anode: Cℓ2(g)
- At the cathode: Zn(s)
c). Electrolysis of water:
Water is a weak electrolyte (weak electric conductor). A small amount of dilute sulphuric acid (H2SO4) is added to water to increase its conductivity. When an electric current is passed through the acidified water, oxidation and reduction reactions take place.
At the anode:
oxidation half-reaction: 2H2O → O2(g) + 4H+ + 4e– E0 = +1,23 V
At the cathode:
reduction half-reaction: 2H+ + 2e– → H2(g) E0 = 0,00 V
The net reaction: 2H2O(ℓ) → 2H2(g) + O2(g)
5.5 Application of electrolysis
- Electroplating
- Production of chemicals e.g. chlorine gas, hydrogen gas and sodium hydroxide (membrane cell, chlor-alkali industry)
- Extraction of metals e.g. aluminium
- Refining (purifying) of metals e.g. copper.
a). Electroplating
Electroplating is the process of putting a metallic coating on an object using electrolytic reactions.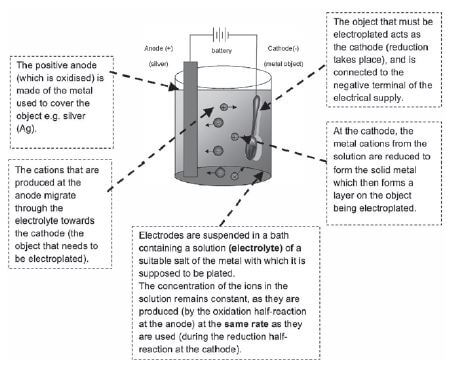
Electroplating is used to protect metals that oxidise easily, by covering them with a thin layer of a metal that does not oxidise easily e.g. chromium, silver or gold. A relatively cheap metal is covered by an expensive metal. Silver is used to cover cutlery — it is too expensive to make a spoon of pure silver, and it is too weak to use — while chromium can be used to cover car parts like bumpers.
e.g. Worked example 4
An attractive silver appearance can be created by electroplating artefacts (objects) made from cheaper metals, such as nickel, with silver. The simplified diagram here represents an arrangement that can be used to electroplate a nickel artefact with silver.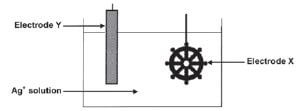
- Which electrode (cathode or anode) does the nickel artefact represent?
- Name the metal represented by electrode Y.
- Write down the half-reaction responsible for the change that occurs at the surface of the artefact.
- Give a reason why the concentration of the electrolyte remains constant during electroplating.
- In industry some plastic articles are sometimes electroplated. Explain why plastic must be coated with graphite before electroplating.
- Give a reason why, from a business point of view, it is not advisable to plate platinum with silver.
Solutions
- Cathode
- Silver
- Ag+ + e– → Ag
- The rate of oxidation of silver at the anode is equal to the rate of reduction of silver ions at the cathode.
- Plastic is a non-conductor. It must be covered with a conducting layer so that it can act as the cathode. Graphite is a conductor.
- Platinum is expensive and is more durable than other metals. You would normally electroplate a cheap metal with an expensive, durable metal and not the other way round.
Activity 3
- TRUE or FALSE? During electroplating of a steel teaspoon with silver, the teaspoon is the cathode and the electrolyte is a solution of any soluble compound [2]
Solution
- FALSE. ✓… the electrolyte is a solution of a soluble silver compound. ✓ [2]
b) Refining of copper
Copper which is mined is impure and the copper ore can be refined as follows by means of electrolysis:
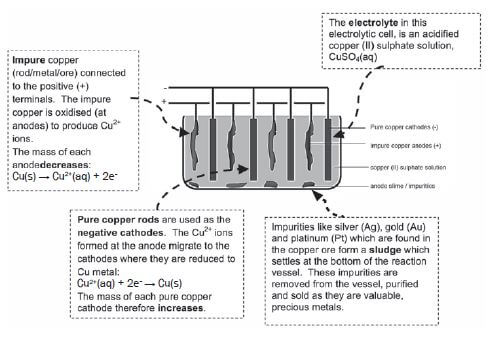
e.g. Worked example 5
Impure copper can be purified by the process of electrolysis. The simplified diagram represents an electrolytic cell used to purify copper.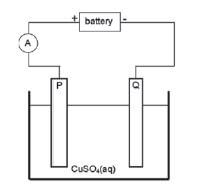
- Define the term electrolysis.
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer.
- Write down the half-reactions that take place at electrodes P and Q.
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process.
- Explain why the concentration of the copper (II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum.
- Why is the sludge of economic importance?
Solutions
- Electrolysis is a process during which electrical energy is converted to chemical energy. It is the process in which electricity is used to bring about a chemical change / decompose / break compounds into components.
- P: P is the positive electrode / anode. The impure Cu is oxidised at the positive electrode / anode.
- P: Anode: Cu(s) → Cu2+(aq) + 2e– oxidation impure
Q:Cathode: Cu2+(aq) + 2e– → Cu(s) reduction pure - Platinum and silver are both weaker reducing agents than copper and will not be oxidised to form ions.
- The rate at which copper is oxidised (at the anode) is equal to the rate at which copper ions are reduced (at the cathode).
- Silver and platinum are valuable and expensive metals and can therefore be sold at a profit.
Activity 4
- Refer to the diagram in the worked example above. One of the electrodes consists of impure copper and the other one of pure copper.
- What type of power source is used to drive the reaction in this cell? Write down only AC or DC. (1)
- Give a reason why the copper(II) sulphate is dissolved in water before it is used in this cell. (1)
When an electric current passes through the solution, electrode P becomes coated with copper. - Is electrode P the cathode or the anode? Support your answer by writing the half-reaction that takes place at electrode P. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
It is found that the impure copper plate contains platinum. The platinum forms a residue at the bottom of the container during electrolysis. - Refer to the relative strengths of reducing agents to explain why platinum forms a residue at the bottom of the container. (2)
- How will the concentration of the copper(II) sulphate solution change during electrolysis? Write down only INCREASES, DECREASES or REMAINS THE SAME. (3) [11]
Solutions
- DC ✓ (1)
- Free ions needed to conduct electricity ✓ (1)
- Cathode. ✓ Cu2+ + 2e– → Cu ✓ (2)
- Cu → Cu2+ + 2e– ✓✓(2)
- Pt is a weaker reducing agent than Cu ✓and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent than Pt and will be oxidised - Remains the same.✓ The rate at which Cu is oxidised at the anode ✓ equals the rate at which Cu2+(aq) is reduced at the cathode ✓ (3) [11]
c) The recovery or extraction of aluminium metal from bauxite
NB: A very important application of electrolysis is the recovery of aluminium. South Africa imports bauxite, an aluminium ore.
Aluminium:
- is one of the most abundant metals on earth, yet it is expensive – largely because of the amount of electricity needed to extract it.
- has the following properties: a low density; the ability to resist corrosion; is very ductile; can be rolled out in thin layers; is lightweight and is a good electrical conductor.
The process involves:
- Aluminium oxide (Aℓ2O3) is extracted from the bauxite and it is then heated.
- The melting point of Aℓ2O3 is higher than 2 000°C. Cryolite (Na3AℓF6) is added to the ore before it is heated. Cryolite does not lower the melting point of Aℓ2O3 but dissolves it. Aℓ2O3 dissolved in a molten cryolite can be electrolysed easily. This means that less electricity is required to extract the aluminium, thereby decreasing the extraction cost.
- The ions, Aℓ3+ and O2–, are formed during the extraction.
- The mass of the anode therefore gradually decreases. The cost of replacing the anode when it is depleted adds to the high cost of the aluminium extraction.
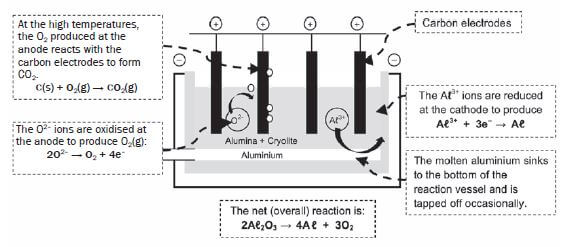
- The ecological impact of Aℓ extraction: Loss of landscape due to the size of the chemical plant needed; disposal of red mud (iron (III) oxide) formed during extraction of aluminium oxide from bauxite, into rivers and lagoons and into ground water.
- Environmental impact of Aℓ extraction: Carbon dioxide from the burning of the anodes contributes to the greenhouse effect, causing global warming. Fluorine and its compounds lost from the cryolite during the electrolysis process are poisonous. Chemicals in the red mud dams drain into the soil and contaminate groundwater. Pollution caused by power generation (for electrolytic process) using coal fired plants leads to acid rain and adds to the greenhouse effect. Noise pollution from the extraction plant.
Activity 5
Give ONE word for the following phrase:
- The main ore from which aluminium is extracted. (1)
- The name of the chemical substance in which Aℓ2O3 is dissolved to lower its melting point during the industrial extraction of aluminium. (1) [2]
Solutions
- Bauxite ✓
- Cryolite ✓ [2]
Activity 6
- In an aluminium smelter, aluminium metal is extracted from bauxite, a hydrated aluminium oxide, via an electrolytic process.
1.1 Write down the energy conversion that takes place in an electrolytic cell. (2)
1.2 Write down the equation for the half reaction responsible for the formation of aluminium metal in a smelter. (2)
1.3 Explain in terms of the relative strength of oxidizing agents why the electrolytic production of aluminum requires more electrical energy than that of iron or copper. (2)
1.4 Name TWO advantages that the use of aluminium has over that of iron. (2) - A huge aluminium smelter is planned for Coega in the Eastern Cape. When operational, it will consume 1350 MW of electricity, or 4% of the nation’s total electrical energy. It is estimated that 5200 jobs will be created at the peak of construction. About 1000 workers will be employed on a full-time permanent basis, and between 200 and 300 full-time subcontractors will also be directly associated with the smelter. (Source: www.engineeringnews.co.za; www.groundwork.org.za).
2.1 Taking the present South African socio-economic realities into account, give ONE reason why the aluminium smelter should:- Not be built (1)
- Be built (1)
2.2 Give ONE reason why environmental activists oppose the construction of the smelter. (1) [11]
Solutions
1.1 Electrical energy → chemical energy ✓✓ (2)
1.2 Aℓ3+ + 3e– → Aℓ ✓✓ (2)
1.3 Aluminium has a lower reduction potential (–1,66 V) ✓/ Weaker oxidizing agent compared to that of iron (–0,44 V) [and copper (+0,34 V)]. The aluminium ions therefore require a large amount of energy to be reduced/ will reduce more difficultly than iron (and copper). ✓ (2)
1.4 It is much lighter for the same strength (or stronger for the same mass). ✓ It is corrosion free.✓ (2)
2.1
- It will consume huge amount of electricity. ✓/ (1) Will cause power failures ✓ (any 2)
- It will create jobs ✓/Create foreign investment. ✓ (1) Contribute to GDP ✓ (any 3)
2.2 The production of the large amount of electricity used ✓ enhances the greenhouse effect (or climate change) ✓ OR The process is responsible for toxic fluoride waste OR pollution. (any one) (1) [11]
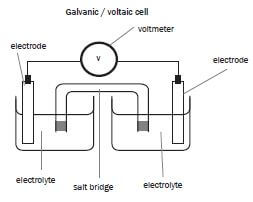
5.6 Voltaic (Galvanic) cells
Remember:
A Galvanic or voltaic cell is a cell in which chemical energy is converted to electrical energy spontaneously. We therefore use a chemical reaction to produce electricity. E.g. standard AA / penlight batteries.
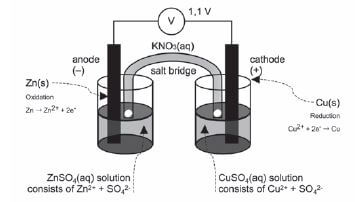
Consider a copper-zinc cell:
A strip of zinc metal is placed in a zinc ion solution. A strip of copper is placed in a separate beaker in an aqueous copper (II) ion solution. These solutions are the electrolytes. As they contain ions which dissociated when the salts were dissolved in water, they are good electric conductors.
- The half reactions that occur in each half cell:
Zn(s) → Zn2+ + 2e– Cu2+ + 2e– → Cu(s)
REMEMBER
- When redox half-reactions are to be written, the correct single arrow should be used.
| Zn half-cell | Cu half-cell |
| Oxidation half-reaction at the anode which is always the negative electrode (e– are given off) | Reduction half-reaction at the cathode which Is always the positive electrode. |
The stronger reducing agent is oxidised (gives off electrons) | The stronger oxidising agent is reduced (receives electrons) |
Zn(s) → Zn2+ + 2e– (read from right to left) | Cu2+ + 2e– → Cu(s) (read from left to right) |
| Zn is the stronger reducing agent | Cu2+ is the stronger oxidising agent |
The anode always decreases in mass if the reducing agent is a solid (The Zn rod is corroding). | The cathode always increases in mass if the reduced product is a solid (copper deposits on rod). |
Net cell reaction:
| |
NB
- In redox reactions, most learners struggle to understand the difference between the name of the process, such as oxidation and the substance, for example the reducing agent.
- Be very careful not to write reduction agent instead write reducing agent.
- Be able to clearly distinguish between the oxidising and the reducing agent.
a). Salt bridge – part of electrochemical cell (usually a tube) containing electrolyte providing electrical contact between two solution.
The salt bridge:
- Completes the circuit.
- Keeps the two electrolytes in the two half-cells separate so that they do not mix.
- Allows movement of ions between the electrolytes, so as to ensure electrical neutrality i.e. it acts as an ion exchanger.
- Contains a saturated salt solution (not 1 mol⋅dm–3) of either KNO3 or KCℓ.
- The electrolyte in the salt bridge must contain ions which are weak reducing agents and weak oxidising agents. This will ensure that these ions are not oxidised or reduced, but act as spectator ions.
NB: Potassium chloride is not suitable for a silver half-cell, because AgCℓ is formed and this compound is insoluble, thus a precipitate will form
b). Electrolyte:
- The salt of the compounds need to be soluble.
- Suitable soluble salts of zinc and copper are zinc nitrate and copper nitrate.
- The electrolyte solutions in this cell is Zn(NO3)2(aq) and Cu(NO3)2(aq).
- As the Zn(s) is oxidised to Zn2+(aq), the concentration of the Zn2+ ions increases in the diagram above. This means that the Zn half-cell will start building up a positive charge. As the electrons will only move away from a negative potential, the function of the cell would become impaired.
- In the same way as the Cu2+ ions are reduced to Cu(s), the concentration of the Cu2+ ions decreases. This leads to the copper half-cell becoming less positive. The electrons are therefore not attracted to the cathode as strongly and the function of the cell would become impaired (weaker).
- The migration of the NO3– anions in the salt bridge to the anode (the Zn half-cell) and of the K+ ions in the salt bridge to the cathode (the Cu half-cell), cancel this build-up of undesired charge and maintain the cell’s electric neutrality. The cell therefore continues to function properly.
NB: The nitrate salts of ionic compounds are very suitable as an electrolyte, since the salts are soluble in water.
c). Electron current:
- Electrons always flow from the anode, through the external circuit, to the cathode.
- In the Cu/Zn cell, electrons therefore flow from the zinc (Zn) to the copper (Cu).
5.7 Cell notation
The structure of the galvanic cell may also be represented in symbols.
Rules:
- Always start with the anode on the left and end with the cathode on the right.
- Use a / to separate the anode or cathode from its electrolyte.
- Represent the salt bridge with the symbol //.
- Inert electrodes (usually Pt or C) and phases are usually included:
Zn(s) / Zn2+(aq) // Cu2+(aq) / Cu(s)
electrode / electrolyte // electrolyte / electrode
anode (–) salt bridge cathode (+) - e.g. Pt/reducing agent/oxidised species//oxidising agent/reduced species/Pt
Pt / Cℓ–(aq) / Cℓ2(g) // F2(g) / F–(aq) / Pt
Activity 7
Give ONE word for the following phrase:
- The electrode in a galvanic cell at which reduction occurs. (1)
- The component of a galvanic cell that allows for the movement of ions between the half-cells. (1)
- Which statement is CORRECT for a Zn-Cu galvanic cell that operates under standard conditions? (Standard Conditions are defined in the tables at the beginning of this book)
- The concentration of the Zn2+ ions in the zinc half-cell gradually decreases.
- The concentration of the Cu2+ ions in the copper half-cell gradually increases.
- Negative ions migrate from the zinc half-cell to the copper half-cell.
- The intensity of the colour of the electrolyte in the copper half-cell gradually decreases. (2)
- The reactions below occur in two different electrochemical cells X and Y.
Cell X: CuCℓ2(aq) → Cu(s) + Cℓ2(g)
Cell Y: Zn(s) + CuSO4(aq) → Cu(s) + ZnSO4(aq)
Which ONE of the following correctly describes the substance that forms at the CATHODE of each of these cells?
Cell X | Cell Y | |
| A | Cℓ2(g) | Cu(s) |
| B | Cu(s) | Cu(s) |
| C | Cℓ2(g) | ZnSO4(aq) |
| D | Cu(s) | ZnSO4(aq) |
5. Which one of the following statements regarding a copper-silver galvanic cell is TRUE?
- Silver is formed at the anode
- Copper is formed at the anode
- Silver is formed at the cathode
- Copper is formed at the cathode (2) [8]
Solutions
1. Cathode ✓ (1)
2. Salt bridge ✓ (1)
3. D ✓✓ (2)
4. B ✓✓ (2)
5. C ✓✓ (2) [8]
5.8 Standard electrode potentials
The Reactivity Series is a list of substances which are arranged in order of their ability to act as reducing agents or as oxidising agents.
The Table of Standard Reduction Potentials lists the standard electrode potentials (E0 values) for various compounds. There are two tables, they are similar, but the entries are arranged in opposite directions.
- The tables of Standard Reduction Potentials can be used to:
- identify oxidising and reducing agents
- write balanced redox reaction equations
- predict whether a redox reaction takes place spontaneously or not
- calculate the emf of a voltaic cell by using one of the following formulae (formulas):
NB
- You will notice that all ionic compounds, for example, H2SO4, HNO3 and HCℓ must be dissociated into ions, before we can use the information in the Table of Standard Reduction Potentials.
E0cell = E0cathode – E0anode OR E0cell = E0oxidising agent – E0reducing agent
REMEMBER
- The formula must be written without any abbreviation in the subscript.
NB
- Further information is discussed on the Table of Standard Electrode Potentials (shown here so you know which table to look for). This table is given at the beginning of this book.
E.g. Stronger reducing agents displace weaker reducing agents, and remember to always start with the oxidation half-reaction. Please read that information now.
e.g. Worked example 6
A piece of zinc metal (Zn) is placed in a beaker containing a copper (II) sulphate solution and a piece of copper metal (Cu) is placed in a beaker containing a zinc (II) sulphate solution.
- Predict which redox reaction will take place spontaneously.
- Motivate your answer.
- Write the oxidation half-reaction, the reduction half-reaction and the net cell reaction for the spontaneous redox reaction.
Solutions
- Zn is oxidised to Zn2+ and Cu2+ is reduced to Cu. The redox reaction in the beaker containing the piece of zinc (Zn) and the copper (II) sulphate solution with Cu2+ ions is spontaneous.
- Zn is a stronger reducing agent than Cu (see reducing ability on table), i.e. Zn will be oxidised more readily than Cu and Cu2+ is a stronger oxidising agent than Zn2+ (see oxidising ability on table) i.e. Cu2+ will be reduced more readily than Zn2+. Therefore Zn will displace Cu from CuSO4(aq).
- oxidation half-reaction : Zn(s) → Zn2+(aq) + 2e–
reduction half-reaction: Cu2+(aq) + 2e– → Cu(s)
Overall or net ionic reaction is: Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
e.g. Worked example 7
- Use the Table of Standard Reduction Potentials to determine whether Ag will spontaneously displace Zn2+ ions from a zinc sulphate (ZnSO4) solution. Motivate your answer.
- Write the oxidation half-reaction, the reduction half-reaction and the net ionic reaction which takes place when a piece of copper (Cu) is dipped in a silver nitrate (AgNO3) solution.
Solutions
- No, the reaction will not take place.
Ag is a weaker reducing agent than Zn, therefore it will not be able to displace Zn from the zinc sulphate solution. - oxidation half-reaction: Cu → Cu2+ + 2e–
reduction half-reaction: Ag+ + e– → Ag+
Multiply this reaction by 2, so that the number of electrons released by the copper atom equals the number of electrons gained.
2Ag+ + 2e– → 2Ag+
net ionic reaction: Cu + 2Ag+ → Cu2+ + 2Ag+
Activity 8
- Which one of the following solutions can be stored in an aluminium container?
(Use the Table of Standard Reduction Potentials.)- CuSO4(aq)
- ZnSO4(aq)
- NaCℓ(aq)
- Pb(NO3)2(aq) (2) [2]
Solution
1. C ✓✓[2]
Activity 9
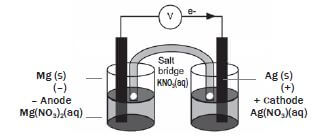
The galvanic cell represented in the diagram consists of a Mg electrode dipped into a Mg(NO3)2 solution, and a Pb electrode dipped into a Pb(NO3)2 solution. Assume that the cell operates under standard conditions.
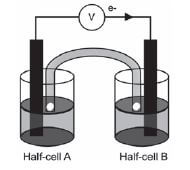
1. State TWO standard conditions under which this cell operates. (2)
2. Write down the half-reaction that takes place in half-cell A. (2)
3. Write down the cell notation for this cell. (2)
4. Calculate the emf of this cell. (3)
5. How will each of the following changes influence the value of the cell’s emf calculated in QUESTION 2.4? Write down only INCREASES, DECREASES or REMAINS THE SAME
5.1 An increase in [Mg2+(aq)] (1)
5.2 An increase in [Pb2+(aq)] (1)
6. In which direction, from half-cell A to B or from half-cell B to A, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer. (3) [14]
Solutions
1. Temperature: 298 K (25 °C) ✓✓(2)
2. Mg(s) → Mg2+(aq) +2e– ✓✓ (2)
3. Mg(s)/Mg2+(1 mol·dm–3) ✓// Pb2+(aq)(1 mol·dm–3)/Pb(s) ✓ (2)
4. E0cell = E0cathode – E0anode = - 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.
5.1 DECREASES ✓ (1)
5.2 INCREASES ✓ (1)
6.
- Half-cell A to half-cell B ✓
- Concentration of positive ions/cations ✓/ Pb2+ ions decreases in half-cell B. ✓
OR - Concentration of positive ions/cations/Mg2+ ions increases in half-cell A
- To prevent a build-up of positive ions in half-cell A and negative ions in half-cell B /for electrical neutrality.
- Positive ions migrate from/through the salt bridge (3) [14]
Activity 10
Learners conduct an investigation to determine which combination of two half-cells will provide the largest emf under standard conditions.
Three half-cells, represented as A, B and C in the table below, are available.
Half-cell A | Half-cell B | Half-cell C |
Mg/Mg2+ | Pb/Pb2+ | Aℓ/Aℓ3+ |
The learners set up galvanic cells using different combinations of the above half-cells.
- Write down the standard conditions under which these cells operate. (2)
- Write down the dependent variable in this investigation. If you can’t remember what the dependent (and independent) variables are, please see the introductory materials at the beginning of this book. (1)
- Use the Table of Standard Reduction Potentials to determine which one of the three half-cells (A, B or C) contains the:
3.1 Strongest reducing agent. (1)
3.2 strogest oxidizing agent. (1) - Without any calculation, write down the combination of two half-cells which will produce the highest emf. Write down only AB, BC or AC. (1)
- One group of learners set up a galvanic cell using half-cells A and B, as shown below. X is one of the components of the galvanic cell.

5.1 Write down the NAME or SYMBOL of the substance that will act as the anode in this cell. Give a reason for the answer. (2)
5.2 Calculate the initial emf of this cell. (3)
5.3 How will an increase in the concentration of the electrolyte in half-cell B affect the initial emf of this cell? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
5.4 Briefly explain how component X ensures electrical neutrality while the cell is functioning. (1) [13]
Solutions
- Temperature 25 °C /298 K ✓
Concentration of electrolytes = 1 mol·dm–3 ✓ (2) - emf/potential difference ✓ (1)
- 3.1 Half-cell A ✓ (1)
3.2 Half-cell B ✓ (1) - Combination AB ✓ (1)
- 5.1 Magnesium/Mg. 3Is oxidised/loses electrons/increase in oxidation number/stronger reducing agent ✓ (2)
5.2 E0cell = E0cathode – E0anode = – 0,13 – (–2,36) ✓ =2,23V✓ (3)
5.3 Increases ✓ (1)
5.4 Allows for the migration of positive ions to the cathode half-cell ✓ (1)
OR
Allows for the migration of negative ions to the anode half-cell [13]
5.9 The standard hydrogen electrode
The hydrogen gas/hydronium ion electrode has been chosen as standard half-cell.
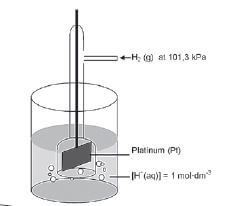

Activity 11
- Which is the the reference electrode in the diagram above? (1)
- TRUE or FALSE? A standard cell that has a negative emf cannot be used as a galvanic cell. (2)
- TRUE or FALSE? The standard conditions used to measure standard electrode potentials are: A temperature of 273 K, a concentration of 1 mol·dm–3, and a pressure of 101,3 kPa. (2)
- Which ONE of the following containers can be used to store an iron(II) sulphate solution?
- Aℓ
- Mg
- Ni
- Zn (2) [7]
Solutions
- Hydrogen half-cell ✓ (1)
- TRUE ✓ (2)
- FALSE. ✓ A temperature of 298 K (25 °C) — the question gives the temperature incorrectly ✓(2)
- C ✓ (2) [7]
NB
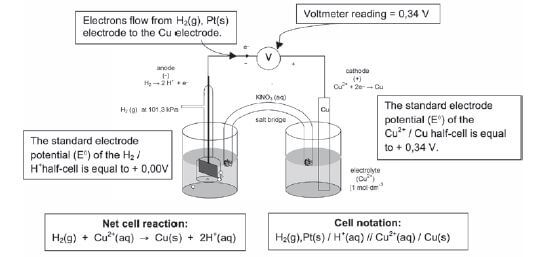
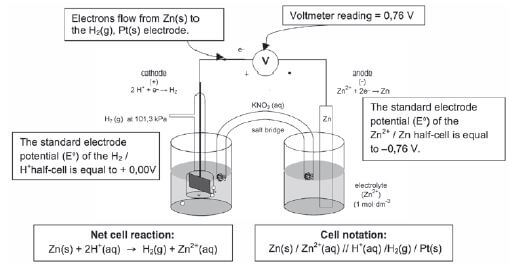
- A half-cell potential of 0,00 V has been assigned to the standard hydrogen electrode. The half-cell potential values are given on the right hand side of the table.
- To determine the standard electrode potential of the copper half-cell

- To determine the standard electrode potential of the zinc half-cell

5.10 The emf of an electrochemical cell
- A voltmeter connected to the zinc / copper cell has a reading of 1,1 V. It reads the difference between the electric potentials of the two separate electrodes.
- This reading of 1,1 V can also be referred to as the potential difference or cell potential or emf of the cell.
e.g. Worked example 8
- Using the Cu / Zn cell as an example:
E0cell = E0cathode – E0anode
+0,34 – (–0,76) = +0,34 + 0,76
+1,1 V
NB
- Always use the values exactly as they stand on the table; do not round off.
- The cell potential (voltage) does not depend on the surface area of the electrode.
- The bigger the surface area of the electrode, the bigger the current produced by the cell.
- In general the cell potential increases slightly with an increase in temperature.
- The reaction in the cell reaches equilibrium when the cell is completely discharged (flat or dead). This usually means that the concentration of the cations in the reduction half-cell (i.e. at the cathode) has decreased to zero.
Using the Table of Standard Reduction Potentials to predict whether a redox reaction will be spontaneous or not:
- Assume that the reaction will take place as suggested in the question.
- Identify the reducing agent and the oxidising agent according to the given equation.
- Calculate the cell potential for this reaction.
- If the cell potential is positive, the reaction will be spontaneous i.e. it will take place without the addition of any other form of energy. If it is negative, the reaction will not occur spontaneously.
- If the two half reactions are very close to each other on the Table of Standard Reduction Potentials, heating the reaction mixture may supply enough additional energy for the reaction to take place.
Steps
- Step 1. Use the Reduction Potentials to determine whether a reaction will take place spontaneously.
- Step 2. If the calculated cell potential is a positive value the redox reaction will take place spontaneously.
NB:
H2S → S + 2H+ + 2e–
OR
S + 2H+ + 2e– ← H2S- is the only correct way of writing a half-reaction
- Note the direction of the single arrow changes, depending on whether the reaction is an oxidation or reduction half reaction.
- Please do NOT include the double arrow in the half reaction.
- Always remember to include the charges on the ions in the half-cell reactions.
NB
When you have to explain the relative strength of oxidizing and reducing agents, write the explanations as follows:
- Cu is a stronger oxidising agent than Mg. Do NOT state the position of a substance in terms of its position in the Reduction Table (e.g. Cu is above Mg)
- Do NOT state the relative strength in terms of relative reactivity only, for example, Mg is more reactive than Cu.
Your correct answer would read as follows:
- Mg is a stronger reducing agent than Cu and therefore Mg will be able to reduce Cu2+ ions to Cu.
OR
Explain the relative strength of the oxidizing and reducing agents in terms of their relative strength as electron acceptor and donors.
- Mg is a stronger reducing agent than Cu because Cu has a stronger tendency to accept electrons than Mg. OR: Mg has a stronger tendency to donate electrons to Cu than Cu has to donate electrons to Mg.
e.g. Worked example 9
Batteries consist of one or more galvanic cells. A galvanic cell is a combination of two half-cells. John wants to determine which one of Options A or B, shown below, can be used to assemble a galvanic cell with the highest potential difference.
| Option | Combination of half-cells |
| A | Ag(s) in AgNO3(aq) & Ni(s) in Ni(NO3)2(aq) |
| B | Mg(s) in Mg(NO3)2(aq) & Ag(s) in AgNO3(aq) |
- Draw a fully labelled diagram of the galvanic cell that John can use to measure the potential difference for the cell in Option B. Use a positive (+) and negative (–) sign to indicate the positive and negative electrodes respectively.
- Write down the oxidation and reduction half reactions as well as a balanced chemical equation, excluding spectator ions, for the net (overall) cell reaction for the galvanic cell in Option B.
- Calculate the initial potential difference that can be obtained under standard conditions for the galvanic cell in Option B.
- State TWO standard conditions that John must adhere to during the experiment, to ensure that the measured potential difference is the same as the calculated potential difference.
- Write down the cell notation for the galvanic cell in Option A.
- Without doing any calculations, determine which one of Option A or Option B should result in the galvanic cell with the highest potential difference. Refer to the relative strengths of the two reducing agents, as well as the two oxidising agents involved, to explain your answer.
- How will each of the following changes influence the value of the cell’s emf calculated in Question 3?
7.1 An increase in [Mg2+(aq)].
7.2 An increase in [Ag+(aq)]. - In which direction, from half-cell Mg2+ / Mg to Ag + / Ag, or from half-cell Ag + / Ag to Mg2+ / Mg, do cations move within the salt bridge to maintain electrical neutrality? Explain how you arrived at your answer.
- Give two functions of the salt bridge.
- Name the cathode and the anode for the galvanic cell in option B.
- Which electrode will show an increase in mass for the galvanic cell in option B?
- Distilled water is added to the Ag+ solution for the galvanic cell in Option B. How will the emf of the cell be influenced? Explain your answer.
- Consider the galvanic cell in Option B. If 0,6 moles of electrons flow from the anode to the cathode, what will the decrease in mass of the anode be?
- A 2 V bulb is connected to the cell in option B instead of the voltmeter. Will the bulb light up? Justify your answer.
- Consider the galvanic cell in Option B. It is observed that after a while, the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell.
- Give two uses of galvanic cells.
Solutions
-

- oxidation: Mg(s) → Mg2+(aq) + 2e–
reduction: Ag+(aq) + e– → Ag(s)
net reaction: Mg(s) + 2Ag+(aq) → Mg2+(aq) + 2 Ag(s) - E0cell = E0cathode – E0anode = 0,80 – (–2,36) = 3,16 V
- Ensure a temperature of 25 °C and Mg2+, Ni2+ and Ag+ solutions of concentration 1 mol·dm3.
- Ni(s) / Ni2+(aq) (1 mol·dm–3) // Ag(s)+(aq) (1 mol·dm–3) /Ag(s)
- Option B. The reaction leading to the highest emf (or potential difference) will be between the strongest reducing agent (Mg) and the strongest oxidising agent (Ag+).
- An increase in the rate of the forward reaction will increase the emf of a galvanic cell.
7.1 decreases; the reverse reaction is favoured
7.2 increases; the forward reaction is favoured - Half-cell Mg2+ / Mg to Ag + / Ag
Concentration of positive ions or cations; [Ag+] ions decreases in half-cell Ag + / Ag. OR
Concentration of positive ions or cations; [Mg2+] ions increases in half-cell Mg2+ / Mg.
To prevent a build-up of positive ions in half-cell Mg2+ / Mg and negative ions in half-cell Ag+ / Ag.
For electrical neutrality, positive ions migrate from/through the salt bridge to the cathode. - Any two: It completes the circuit. It ensures electrical neutrality by allowing the migration of ions through the salt bridge. It separates the electrolytes.
- cathode = Ag anode = Mg
- cathode = Ag
- By adding water to the solution, the [Ag+] will decrease. Thus the reverse reaction is favoured and emf decreases.
- Mg(s) → Mg2+(aq) + 2e– (anode)
From the balanced equation 1 mol Mg → 2 mol e–
thus 0,3 mol Mg → 0,6 mol e–
n = m/M
molar mass (M) of Mg = 24 g·mol–1
m = n × M
m = 0,3 × 24 = 7,2 g
mass loss is 7,2 g - The bulb will light up, as the emf of the cell is 3,16 V which is more than the required 2 V.
- While the cell is in operation, the concentration of the cations in the reduction half-cell Ag+(aq) decreases. At the same time, the concentration of the cations in the oxidation half-cell Mg2+(aq) increases. The result is a gradual decrease in the cell potential until there is no further change in the concentration and equilibrium is reached, and the cell potential will be zero.
- Any two: Torch cells, car batteries, batteries in toys and small appliances like remote controls.
e.g. Worked example 10
Rusting is an unwanted redox reaction. Iron rusts when exposed to oxygen and moisture. The unbalanced ionic equation for one reaction that occurs during rusting is represented below.
Fe(s) + O2(g) + H2O(ℓ) → Fe2+(aq) + OH–(aq)
Use the Table of Standard Reduction Potentials to answer the following questions for this reaction:
- Write down the oxidation and the reduction half-reactions.
- Write down the NAME of the substance that is reduced.
- Perform a calculation to verify that this reaction is spontaneous.
Solutions
- oxidation: Fe → Fe2+ + 2e–
reduction: 2H2O + O2 + 4e– → 4OH– - oxygen
- oxidation: Fe → Fe2+ + 2e– E0anode = –0,44 V
reduction: 2H2O + O2 + 4e– → 4OH– E0cathode = 0,40 V
E0cell = E0cathode – E0anode = 0,4 – (–0,44) = 0,84 V
Because E0cell is positive, the reaction is spontaneous.
e.g. Worked example 11
Magnesium is used to protect underground iron pipes against rusting (cathodic protection). The diagram here shows an iron pipe connected to a magnesium bar.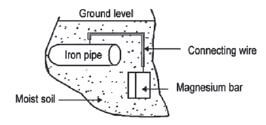
- Use the Table of Standard Reduction Potentials to explain why magnesium can be used to protect an iron pipe against rusting.
- The iron pipe in contact with the magnesium bar forms an electrochemical cell. What serves as the salt bridge of this cell?
- Give a reason why the magnesium bar must be replaced after some time.
- Write down a half-reaction to support your answer to Question 3.3.
- Name TWO other methods that can be used to protect iron pipes against rust.
- State ONE advantage and ONE disadvantage of using plastic pipes instead of iron pipes.
Solutions
- Mg is a stronger reducing agent (than Fe) and will be oxidised more readily than Fe which is a weaker reducing agent.
- The salts which are dissolved in the moist soil.
- Mg is continuously oxidised to Mg2+ and therefore its mass decreases i.e. it is used up.
- Mg → Mg2+ + 2e–
- Any two: Paint; electroplating e.g. galvanising; oil or waterproofing; plastic coating.
- Advantages: Plastic is cheaper; plastic does not rust.
Disadvantages: Plastic not biodegradable; plastic is not as strong as iron.
Activity 12
Give ONE word for the following phrase:
- The process taking place in a cell when an electric current passing through its electrolyte, results in chemical reactions at its electrodes. (1)
- TRUE or FALSE? A battery labelled as 3 000 mA·h can deliver a current of 500 mA for 6 hours. (2) [3]
Solutions
1. Electrolysis ✓ (1)
2. True. ✓✓ (2) [3]
Activity 13
- The most common filling for tooth cavities is ‘dental amalgam’ – a solid solution of tin and silver in mercury. If you bite on a piece of aluminium foil that is in contact with a dental filling in your mouth, you may feel a painful sensation because
- the aluminium foil is hard.
- a temporary galvanic cell has been set up whilst the aluminium and filling are in contact.
- electrons are being transferred to the aluminium.
- a temporary electrolytic cell has been set up whilst the aluminium and filling are in contact. (2)
- Which one of the following can be classified as a redox reaction?
- NH3(g)+ HNO3(g) →NH4NO3(s)
- FeS(aq)+ 2HCℓ(aq) → FeCℓ2(aq) + H2S(g)
- Aℓ(s) + Cℓ2(g) →AℓCℓ3(s)
- Mg(NO3)2(s) → Mg2+(aq) + 2NO3–(aq) [with H2O catalyst] (2)
- A silver (Ag) spoon is left in a beaker containing copper nitrate, Cu(NO3)2, solution. What will be observed after some time?
- The spoon will be covered with a thin layer of copper.
- The nitrate will form NO2 gas and copper will form in the beaker.
- The spoon will start to erode and its mass will decrease.
- There will be no reaction.
Solutions
1. B. ✓✓ (2)
2. C. ✓✓ (2)
3. D. ✓✓ (2) [6]
Activity 14
- A group of learners set up an electrochemical cell using lead and copper half cells.
1.1 Which ONE of copper or lead will be the negative electrode? Give a reason for your answer. (2)
1.2 Use the Table of Standard Reduction Potentials and write down the reduction half-reaction that will take place in this cell. (2)
1.3 A 2V bulb is connected to the cell. Will the bulb light up? Justify your answer with a calculation. (5)
1.4 A voltmeter is now connected to the cell instead of the bulb. It is observed that after a while the reading on the voltmeter drops to zero. We say the cell is ‘flat’ or ‘dead’. Explain this observation in terms of the concentrations of the solutions in the cell. (3) - A cell such as the one described above is not much useful. However, the principle is used in batteries for cars, torches, computers, et cetera. These batteries are called secondary cells. One such battery is the mercury cell. The half reactions occurring in this cell are shown below.
Zn(s) + 2OH–(aq) → ZnO(s) + H2O + 2e–……… (i)
HgO(s) + H2O + 2e– → Hg(ℓ) + 2OH–(aq)….….. (ii)
2.1 Write down the overall cell reaction. (3)
2.2 Why does the use of this battery pose an environmental hazard? (1) [16]
Solutions
1.1 Lead. ✓ Stronger reducing agent ✓ OR Is oxidised preferably (2)
1.2 Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
1.3 E0cell = E0cathode − E0anode ✓ = 0,34– (–0,13) ✓ =0,47V✓ (5) Bulb will not light, ✓ energy from cell not sufficient ✓ OR emf of cell is much less than 2 V needed for the bulb ✓✓
1.4. While the cell is in operation, the concentration of the reactants (Cu2+(aq)) decreases. ✓ At the same time the concentration of the products (Pb2+(aq)) increases. ✓ The result is a gradual decrease in the cell potential until there is no further change in concentration and equilibrium is reached where the cell potential will be zero. ✓ (3)
2.1 Zn(s) + HgO(s) → ZnO(s) + Hg(ℓ) ✓✓✓ (3)
2.2 Mercury is poisonous ✓ (1) [16]
Activity 15
The discovery of electrochemical cells has revolutionised our way of life.
The diagram below represents an electrochemical cell.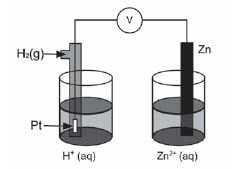
- Name the type of electrochemical cell that converts chemical energy to electrical energy. (1)
- If the electrochemical cell is set up as illustrated, there will be no reading on the voltmeter. Give a reason for this observation.(1)
- Write down the value of the standard emf of the electrochemical cell when it is functioning. (1)
- Write down the voltmeter reading when the net cell reaction in the above electrochemical cell reaches equilibrium. (1)
- Write down the equation for the reaction that occurs at the anode. (1)
- Another electrochemical cell is set up under standard conditions by replacing the standard hydrogen half-cell with a standard magnesium half-cell.
6.1 Which electrode will undergo a decrease in mass? Give a reason for your answer. (2)
6.2 Calculate the initial emf of this electrochemical cell under standard conditions. (3)
6.3 After a while the emf of this electrochemical cell decreases. Explain this observation by referring to the concentration of the electrolytes. (2) - Electrochemical cells such as motor car batteries with plastic casings can harm the environment if not disposed of safely. Suggest TWO ways how motor car batteries can be safely disposed of. (2) [14]
Solutions
1. Galvanic/voltaic cell ✓ (1)
2. Incomplete circuit/No salt bridge ✓ (1)
3. 0,76 V ✓ (1)
4. Zero ✓ (1)
5. Zn(s) → Zn2+(aq) + 2e– ✓ (1)
6.1 Mg. ✓ Mg is oxidised ✓ (2)
6.2 E0cell = E0cathode – E0anode ✓ = 0,76 – (–2,36) ✓ = 1,6 V ✓ (3)
6.3 As the cell functions, the concentration of zinc ions (reactants) decreases ✓ relative to the standard conditions and the concentration of magnesium ions (products) increases relative to standard conditions. The reverse reaction starts opposing the forward reaction causing the emf to decrease relative to standard conditions. ✓ (2)
7. Neutralise acid before disposal. ✓ Recycle plastic casing and lead electrodes ✓ (2) [14]
Activity 16
In 1780, Luigi Galvani discovered that when copper and zinc metal were connected to each other and if each free end touched different parts of the same nerve of a frog leg at the same time, the frog’s leg contracted. He called this “animal electricity”.
- Briefly explain what this “animal electricity” really was. (1)
The diagram shows an electrochemical cell setup under standard conditions using aluminium (Aℓ) and nickel (Ni) electrodes. AℓCℓ3(aq) and NiCℓ2(aq) are used as the electrolytes, and a solution of sodium nitrate (NaNO3(aq)) is used in the salt bridge.
Answer each of the following questions on this electrochemical cell:
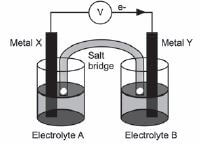
- The diagram indicates that electrons flow from metal X to metal Y.
Identify:
2.1 Metal X (1)
2.2 Electrolyte B (1) - What is the concentration of electrolyte B? (1)
- Write down the FORMULA of the substance that moves towards metal Y in the salt bridge. (1)
- Write down the half-reaction that occurs at the cathode of this cell. (2)
- Calculate the reading on the voltmeter. (3)
- State what happens to the concentration of metal ions in the solution containing electrolyte as time goes by? (1)
- 8.1 Consider 5.7 again and recall your answer. What effect does this have on the voltmeter reading? (1)
8.2 Briefly explain your answer to 5.8.1. (2) [14]
Solutions
- The chemical reaction between the zinc and the copper r eleased electrical energy ✓ (1)
- 2.1 Metal X: Aℓ ✓ (1)
2.2 Electrolyte B: NiCℓ2 ✓ (1) - 1mol·dm–3 ✓ (1)
- NO3– ✓ (1)
- Ni2++ 2e– → Ni ✓ ✓ (2)
- E0cell = E0oxidising agent − E0reducing agent ✓ = –0,25V–(–1,66V) ✓ =1,41V✓ (3)
- Increase ✓ (1)
- 8.1 The voltmeter reading decreases ✓ (1)
8.2 As the [Aℓ3+] increases, ✓ the reverse reaction in the Aℓ Aℓ3+ half-cell:
Aℓ ⇌ Aℓ3+ +3e– is favoured.✓ (2)
OR
The tendency of the net reaction Aℓ + Ni2+ → Aℓ3+ + Ni to proceed from left to right is reduced. This lowers the electrode potential of this half-cell, resulting in a lower cell potential. [14]
Activity 17
Tina wants to investigate the effect of the area of the metal plates used as electrodes in a galvanic cell on the emf of the cell. She sets up the following Zn/Pb cell under standard conditions and measures the emf.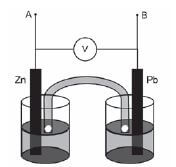
- Which electrode will show an increase in mass when this cell is functioning? (1)
- Write down the equation for the half-reaction occurring at the anode. (2)
- Calculate the emf that you would expect Tina to read on the voltmeter. (3)
- Name TWO variables that should be controlled for during this investigation. (2)
- Tina now replaces the two metal plates with ones of larger surface area, and takes the readings again.
5.1 How would you expect the new emf to compare with the one calculated in Question 6.3? (Only write SMALLER THAN, LARGER THAN or EQUAL TO) (1)
5.2 Explain your answer to Question 5.1 (1) - Tina now connects a resistor of low resistance across terminals A and B. She notes that the reading on the voltmeter immediately drops.
6.1 Give a reason for this observation. (1)
6.2 After some time she observes a further drop in the reading on the voltmeter. Give a reason for this observation. (2) [13]
Solutions
- Pb ✓
- Zn → Zn2+ + 2e– ✓✓
- E0cell = E0oxidising agent − E0reducing agent✓ –0,13 – (–0,76) ✓ =0,63V✓ (3)
- Temperature, ✓ (initial) concentration of electrolytes ✓(2)
- 5.1 Equal to ✓ (1)
5.2 Area/size of electrodes has no effect on the emf of a cell. It is still a standard cell ✓ (1) - 6.1 the cell has internal resistance ✓ (1)
6.2 The emf decreases as the concentration of Pb2+(aq) decreases. ✓/The cell is running flat as the electrolyte concentration in the Pb cell decreases. ✓✓ (any 2) (2) [13]
Activity 18
Electrolysis is an important industrial process used to decompose compounds, extract metals from their ores and to purify metals like gold or copper.
The simplified diagram below represents an electrolytic cell used to purify copper.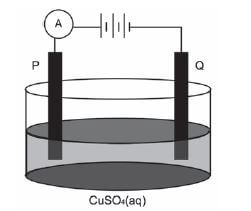
- Define the term electrolysis. (2)
- Which electrode, P or Q, consists of the impure copper? Explain how you arrived at your answer. (2)
- Write down the half-reaction that takes place at electrode Q. (2)
- During purification, metals such as silver and platinum form sludge at the bottom of the container. Refer to the relative strengths of reducing agents to explain why these two metals do not form ions during the purification process. (2)
- Explain why the concentration of the copper(II) sulphate solution remains constant. Assume that the only impurities in the copper are silver and platinum. (2)
- Why is the sludge of economic importance? (1) [11]
Solutions
- The process in which electricity is used to bring about a chemical change / decompose / break compounds into components ✓✓(2)
OR
A process in which electrical energy is converted to chemical energy. - P. ✓
P is the positive electrode /anode ✓ (2)
OR
Oxidation takes place at the positive electrode/anode - Cu2+(aq) + 2e– → Cu(s) ✓✓ (2)
- Pt and Ag are both weaker reducing agents ✓ (than copper) and will not be oxidised ✓ (2)
OR
Cu is a stronger reducing agent (than Pt and Ag) and will be oxidised - The rate at which copper is oxidised at the anode, ✓ is equal to the rate at which copper ions are reduced at the cathode. ✓ (2)
- Contains platinum and silver that are valuable/expensive metals ✓ (1) [11]