PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC EXAMS PAST PAPERS AND MEMOS NOVEMBER 2018
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
PAPER 2
GRADE 12
NSC EXAMS
PAST PAPERS AND MEMOS NOVEMBER 2018
INSTRUCTIONS AND INFORMATION
- Write your examination number and centre number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A-D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 D.
1.1Which ONE of the following is the structural formula of the functional group of the KETONES?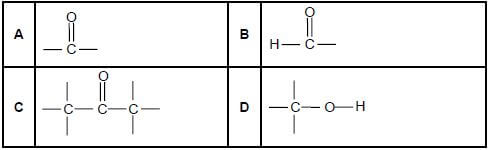 (2)
(2)
1.2 Which ONE of the formulae below represents an ALKANE?
- C2H4
- C5H10
- C14H30
- C8H14 (2)
1.3 Consider the organic compound below.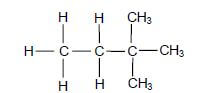
The IUPAC name of this compound is …
- 2,3-dimethyl butane.
- 3,3-dimethyl butane.
- 2,2-dimethyl butane.
- 1,1,1-trimethyl propane. (2)
1.4 Activation energy can best be described as the minimum energy required to …
- cause effective collisions.
- make reactant molecules collide.
- change the orientation of reactant molecules.
- increase the kinetic energy of reactant molecules. (2)
1.5 Which statement is CORRECT for a system in DYNAMIC EQUILIBRIUM?
- All reactants are used up.
- The forward reaction is equal to the reverse reaction.
- All substances in the reaction are of equal concentration.
- The concentration of the reactants and products remain constant. (2)
1.6 Initially, a certain amount of P(g) was placed in an empty container. The hypothetical reaction reaches equilibrium in a closed container according to the following balanced equation:
P(g) ⇌ 2Q(g) ΔH < 0
At time t, the temperature is increased. Which graph below best illustrates the resulting changes in the rates of the forward and reverse reactions after the temperature is increased?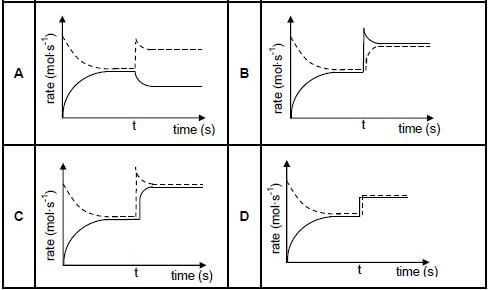
1.7 Reactions I and II below have equilibrium constants (Kc) greater than 1.
- : H3X + HCO-3 ⇌ H2X− + H2CO3 Kc > 1
- : H3O+ + H2X− ⇌ H2O + H3X Kc > 1
Based on the reactions above, the ACIDS in order of INCREASING STRENGTH (weakest to strongest) are …
- H3X, H2X−, H3O+
- H2CO3, H3X, H3O+
- H3X, H2CO3, H3O+
- H3X, H3O+, H2CO3 (2)
1.8 Consider the cell notation for a galvanic cell below.
Ni(s) | Ni2+(aq) || H+(aq) | H2(g) | Pt(s)
Which ONE of the following half-reactions takes place at the ANODE of this cell?
- 2H+(aq) + 2e− → H2(g)
- H2(g) → 2H+(aq) + 2e−
- Ni2+(aq) + 2e− → Ni(s)
- Ni(s) → Ni2+(aq) + 2e− (2)
1.9 Which ONE of the following is applicable to an ELECTROLYTIC CELL?
- Reduction takes place at the anode.
- Oxidation takes place at the cathode.
- It uses alternating current.
- A battery is used for the cell to function. (2)
1.10 The flow diagram below shows four stages (A, B, C and D) in the conversion of sulphur to sulphuric acid.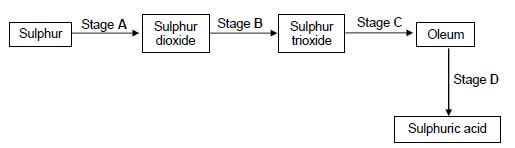
At which stage is a catalyst used?
- A
- B
- C
- D (2) [20]
QUESTION 2 (Start on a new page.)
A test tube containing a straight chain organic acid X, ethanol and a catalyst is heated in a water bath, as illustrated below.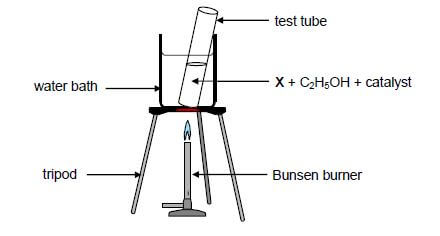
Organic compound Y is produced according to the following equation:
X + C2H5OH ⟶ Y + H2O
2.1 Give a reason why the test tube is heated in a water bath instead of directly over the flame. (1)
2.2 Write down the:
2.2.1 Type of reaction that takes place here (1)
2.2.2 FORMULA of the catalyst needed (1)
2.2.3 Homologous series to which compound Y belongs (1)
The molecular mass of compound Y is 144 g∙mol-1 and its empirical formula is C4H8O.
2.3 Determine the molecular formula of compound Y. (2)
2.4 Write down the IUPAC name of compound Y. (2)
2.5 Write down the structural formula of the organic acid X. (2) [10]
QUESTION 3 (Start on a new page.)
The boiling points of different organic compounds are given below.
COMPOUND | BOILING POINT (°C) | |
A | HCOOH | 101 |
B | CH3COOH | 118 |
C | CH3CH2COOH | 141 |
D | CH3CH2CH2COOH | 164 |
3.1 Define boiling point. (2)
3.2 Write down the:
3.2.1 Name of the FUNCTIONAL GROUP of these compounds (1)
3.2.2 IUPAC name of compound C (1)
3.2.3 Structural formula of the FUNCTIONAL isomer of compound B (2)
3.3 Which ONE of the compounds, A or B or C, has the highest vapour pressure? Refer to the data in the table to give a reason for the answer. (2)
3.4 The boiling point of compound B is now compared with of compound X.
COMPOUND | BOILING POINT (°C) | |
B | CH3COOH | 118 |
X | CH3CH2CH2OH | 98 |
3.4.1 Besides the conditions used to determine boiling points, give a reason why this is a fair comparison. (1)
3.4.2 Is compound X a PRIMARY, SECONDARY or TERTIARY alcohol? Give a reason for the answer. (2)
3.4.3 Fully explain the difference between the boiling points by referring to the types of intermolecular forces present in each of these compounds. (4) [15]
QUESTION 4 (Start on a new page.)
4.1 Three reactions of organic compounds from the same homologous series are shown below.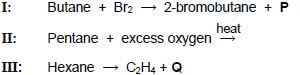
4.1.1 Define a homologous series. (2)
4.1.2 Name the type of reaction represented by I. (1)
4.1.3 Write down the formula of the inorganic compound P. (1)
4.1.4 Give the structural formula of a POSITIONAL isomer of 2-bromobutane. (2)
4.1.5 Using molecular formulae, write down the balanced equation for reaction II. (3)
Reaction III is an example of a cracking reaction.
4.1.6 Define a cracking reaction. (2)
4.1.7 Give the structural formula of organic compound Q. (2)
4.2 Study the flow diagram below.![]()
4.2.1 Write down the IUPAC name of compound R. (2)
4.2.2 Compound R reacts in the presence of concentrated phosphoric acid to form an alkene. Write down the structural formula of the MAJOR PRODUCT in this reaction. (2) [17]
QUESTION 5 (Start on a new page.)
The reaction of zinc and EXCESS dilute hydrochloric acid is used to investigate factors that affect reaction rate. The balanced equation for the reaction is:
Zn(s) + 2HCℓ(aq) ⟶ ZnCℓ2(aq) + H2(g)
The reaction conditions used and the results obtained for each experiment are summarised in the table below. The same mass of zinc is used in all the experiments. The zinc is completely covered in all reactions. The reaction time is the time it takes the reaction to be completed.
EXPERIMENT | CONCENTRATION OF HCℓ (mol∙dm-3) | VOLUME OF HCℓ (cm3) | STATE OF DIVISION OF Zn | TEMPERATURE OF HCℓ (°C) | REACTION TIME (min.) |
1 | 2,0 | 200 | powder | 25 | 7 |
2 | 1,5 | 200 | granules | 25 | 14 |
3 | 5,0 | 200 | powder | 25 | 5 |
4 | 1,5 | 400 | granules | 25 | x |
5 | 2,0 | 200 | powder | 35 | 4 |
5.1 Experiment 1 and experiment 5 are compared. Write down the independent variable. (1)
5.2 Define reaction rate. (2)
5.3 Write down the value of x in experiment 4. (2)
5.4 The Maxwell-Boltzmann energy distribution curves for particles in each of experiments 1, 3 and 5 are shown below.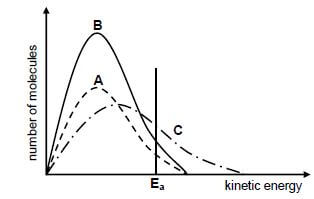
Identify the graph (A or B or C) that represents the following:
5.4.1 Experiment 3 Give a reason for the answer. (2)
5.4.2 Experiment 5 Give a reason for the answer. (2)
5.5 Experiment 6 is now conducted using a catalyst and the SAME reaction conditions as for Experiment 1.
5.5.1 What is the function of the catalyst in this experiment? (1)
5.5.2 How will the heat of reaction in experiment 6 compare to that in experiment 1? Choose from: GREATER THAN, EQUAL TO or LESS THAN. (1)
5.6 Calculate the average rate of the reaction (in mol·min-1) with respect to zinc for experiment 2 if 1,5 g of zinc is used. (4) [15]
QUESTION 6 (Start on a new page.)
Dinitrogen tetraoxide, N2O4(g), decomposes to nitrogen dioxide, NO2(g), in a sealed syringe of volume 2 dm3.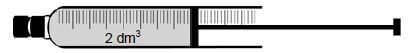
The mixture reaches equilibrium at 325 °C according to the following balanced equation:
- N2O4(g) ⇌ 2NO2 (g)
colourless brown
When equilibrium is reached, it is observed that the colour of the gas in the syringe is brown.
6.1 State Le Chatelier's principle. (2)
6.2 The syringe is now dipped into a beaker of ice water. After a while the brown colour disappears. Is the forward reaction EXOTHERMIC or ENDOTHERMIC? Explain the answer using Le Chatelier's principle. (3)
6.3 The volume of the syringe is now decreased while the temperature is kept constant. How will EACH of the following be affected? Choose from: INCREASES, DECREASES or REMAINS THE SAME.
6.3.1 The number of moles of N2O4(g) (1)
6.3.2 The value of the equilibrium constant (1)
6.3.3 The rate of the forward and reverse reactions (1)
6.4 Initially X moles of N2O4(g) were placed in the syringe of volume 2 dm3. When equilibrium was reached, it was found that 20% of the N2O4(g) had decomposed. If the equilibrium constant, Kc, for the reaction is 0,16 at 325 °C, calculate the value of X. (8) [16]
QUESTION 7 (Start on a new page.)
7.1 Sulphuric acid is a strong acid present in acid rain. It ionises in two steps as follows:
- : H2SO4(aq) + H2O(ℓ) ⇌ H3O+(aq) + HSO-4 (aq)
- : HSO-4 (aq) + H2O(ℓ) ⇌ H3O+(aq) + SO2-4 (aq)
7.1.1Define an acid in terms of the Lowry-Brønsted theory. (2)
7.1.2 Write down the FORMULA of the conjugate base of H3O+(aq). (1)
7.1.3 Write down the FORMULA of the substance that acts as an ampholyte in the ionisation of sulphuric acid. (2)
7.2 Acid rain does not cause damage to lakes that have rocks containing limestone (CaCO3). Hydrolysis of CaCO3 results in the formation of ions, which neutralise the acid.
7.2.1 Define hydrolysis of a salt. (2)
7.2.2 Explain, with the aid of the relevant HYDROLYSIS reaction, how limestone can neutralise the acid. (3)
7.3 The water in a certain lake has a pH of 5.
7.3.1 Calculate the concentration of the hydronium ions in the water. (3)
The volume of water in the lake is 4 x 109 dm3. Lime, CaO, is added to the water to neutralise the acid according to the following reaction:
CaO + 2H3O+ ⇌ Ca2+ + 3H2O
7.3.2 If the final amount of hydronium ions is 1,26 x 103 moles, calculate the mass of lime that was added to the lake. (7) [20]
QUESTION 8 (Start on a new page.)
8.1Corrosion is a redox reaction that takes place in the presence of oxygen and water. Rusting is the corrosion of iron leading to the formation of iron(III) ions.
8.1.1 Define oxidation in terms of electron transfer. (2)
A cleaned copper rod and a cleaned iron nail are placed in a beaker containing water at 25°C, as shown below.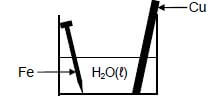
After a while it was observed that the iron nail was coated with rust. The copper rod showed no visible signs of corrosion.
8.1.2Write down the half-reaction for the iron nail. (2)
8.1.3 Does iron act as REDUCING AGENT or OXIDISING AGENT in the beaker? (1)
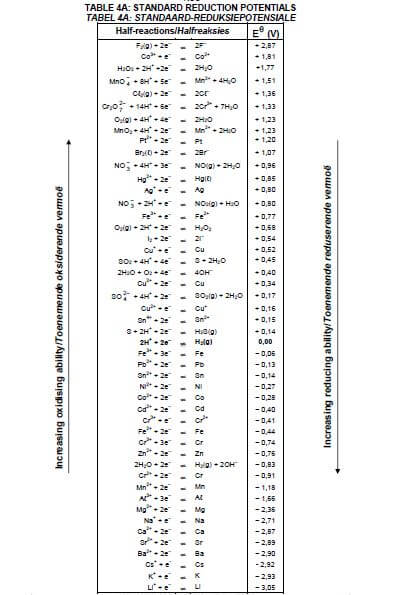
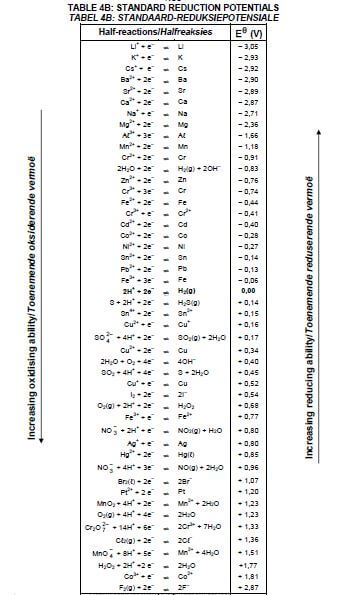
8.1.4 Explain the above observation by referring to the Table of Standard Reduction Potentials. (3)
To prevent rusting of an underground iron pipe, the pipe is connected to a metal (Q) that corrodes easily.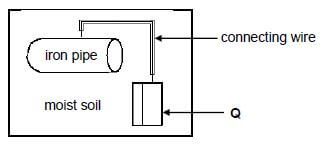
8.1.5 You are given two metals, Zn and Cu, to use as metal Q. Which metal would more suitable? Give a reason. (2)
8.2 A galvanic cell is constructed using a Fe | Fe3+ half-cell and a Cu | Cu2+ half-cell.
8.2.1 Write down the overall (net) cell reaction that takes place when the cell is functioning. (3)
8.2.2 Calculate the cell potential of this cell under standard conditions. (4) [17]
QUESTION 9 (Start on a new page.)
The electrolytic cell below is set up to obtain pure copper from a piece of impure copper.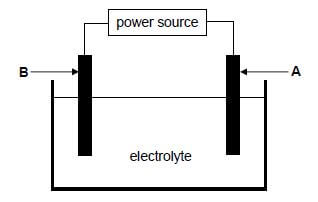
The impure copper contains other metals, such as platinum, iron, cobalt, silver and nickel.
The cell potential of the power source is adjusted so that only copper is deposited on electrode B.
9.1 Define an electrolytic cell. (2)
9.2 Write down the FORMULA of a suitable electrolyte for this cell. (1)
9.3 Which electrode (A or B) is the cathode? Write down the relevant half-reaction taking place at this electrode. (3)
9.4 Sludge forms below one of the electrodes while the cell above is in operation. Which of the metals, PLATINUM, IRON, COBALT, SILVER or NICKEL, will be present in the sludge? (2) [8]
QUESTION 10 (Start on a new page.)
In the flow diagram below, I and II represent industrial processes used in the fertiliser industry. P and Q are chemical reactions that take place to produce ammonium sulphate and fertiliser Y respectively.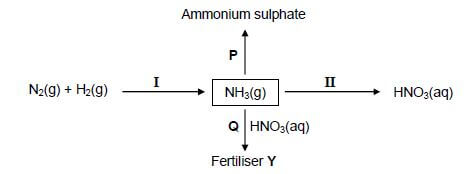
10.1 Write down the name of the industrial process:
10.1.1 I (1)
10.1.2 II (1)
10.2 Write down the NAME or FORMULA of:
10.2.1 Fertiliser Y (1)
10.2.2The catalyst used in process I (1)
10.3 In reaction P, NH3(g) reacts with another substance. Write down a balanced equation for this reaction. (3)
10.4 The following substances are present in a bag of fertiliser:
- 20 kg ammonium nitrate (NH4NO3)
- 12 kg sodium phosphate (Na3PO4)
- 18 kg potassium chloride (KCℓ)
Calculate the NPK ratio of the fertiliser. (5) [12]
TOTAL: 150
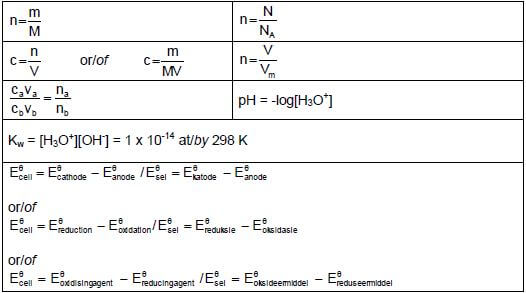
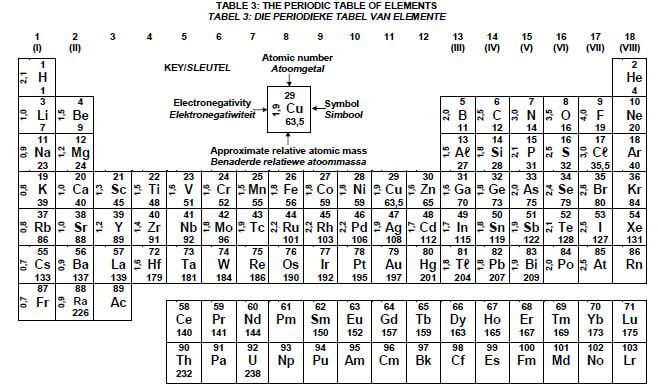
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 x 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 x 10-19 C |
Avogadro's constant | NA | 6,02 x 1023 mol-1 |