OPTICAL PHENOMENA AND PROPERTIES OF MATERIALS - PHYSICAL SCIENCES PAPER 1 STUDY GUIDES AND NOTES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Electromagnetic waves and visible light: Revision
- The photoelectric effect
9.1 Electromagnetic Waves and Visible Light: Revision
9.1.1 Electromagnetic waves
Electromagnetic waves consist of electric and magnetic fields. These fields are perpendicular to each other and to the direction in which the wave is propagated (the direction in which it travels).
You must remember:
- Electromagnetic waves travel through a vacuum at 3 × 108 m·s−1
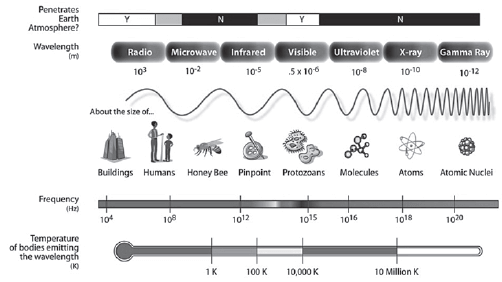
- They increase in frequency and energy from radio waves (lower frequency and less energy) to gamma rays (higher frequency and more energy)
- They increase in wavelength from gamma rays (shorter wavelength) to radio waves (longer wavelength)
- The visible light spectrum is part of the electromagnetic spectrum (shown below).

The electromagnetic spectrum (Source:http://en.wikipedia.org/wiki/Electromagnetic_spectrum)
9.1.2 Visible light
Visible light is part of the electromagnetic spectrum. You must remember:
- Visible light increases in frequency and energy from red (lower frequency, less energy) to violet (higher frequency, more energy)
- Visible light increases in wavelength from violet (shorter wavelength) to red (longer wavelength).
- Visible light has a dual nature because it has wave properties while it is propagated (transmitted) and it has particle properties when it strikes and interacts with other matter (the photoelectric effect).
9.2 The Photoelectric Effect
The photoelectric effect is used in solar panels to generate electricity. The photoelectric effect refers to the ability of light to cause metals to release electrons. You must remember:
- Light energy is transmitted in ‘packages’ which are called photons.
- Each photon consists of a certain amount of energy which is called a quantum.
- The amount of energy (E) in a quantum is directly proportional to the frequency (f) of the light.
- An electron needs a minimum amount of energy to be released from an atom. So the photon providing this energy must have a minimum frequency before it will allow an electron to be released from the metal surface.
- When light shines on a surface (like a metal), the photons collide with the atoms in the surface.
- All the energy of the photon (E = hf) is transferred to the atom with which the photon collides.
- If an electron in an atom on the surface of the metal gains sufficient energy during the collision, it is ejected from the metal surface and is called a photoelectron.
Definitions
- The photoelectric effect is the process whereby electrons are ejected from a metal surface when light of suitable frequency is incident on (falls on) that surface.
- The work function (W0) of a metal is the minimum energy that is required to emit a photoelectron from the surface of the metal.
- The threshold frequency or cut-off frequency (f0) is the minimum frequency of the incident photons (light) that is required to emit a photoelectron from the surface of the metal.
energy ∝ frequency and work function ∝ threshold (cut-off) frequency
∴ E ∝ f and W0 ∝ fo
∴ E = hf and W0 = hfo
NB
Dual nature of light:
- Wave nature during propagation proved by diffraction and interference
- Particle nature during interaction with matter proved by the photoelectric effect
- The formula c = fλ is used to calculate the speed of light, where c (3 × 108 m∙s–1) is the speed of light in metres per second, λ is the wavelength in meters (m) and f is the frequency in hertz (Hz).
- The formula E = hf is used to calculate the energy of radiation, where E is the energy of radiation in Joule (J), h is Planck's constant (6,63 × 10–34 J∙s–1) and f is the frequency in hertz (Hz).
9.2.1 Changing the frequency and intensity of the incident light
- The intensity of a light wave is measured by the power (wattage) of the light source. An 8 W (watt) CFL lamp is dim but a 16 W CFL lamp is bright.
- It also depends on the type of light. So, for example, Light Emitting Diode (LED) lights use very little power but are very bright, Compact Fluourescent Lights (CFLs) use a mid-range amount of power and are bright for the amount of power they use, and Tungsten Filament or Incandescent Lamps use a lot of power for the amount of light they provide E.G. 60 W, 100 W.
- This means that the same type of light with a higher wattage will be brighter than the same type of light with a lower wattage.
| When the frequency of the incident light (the light falling on the metal) is greater than the threshold frequency, changes to the intensity (brightness) and the frequency cause these changes: | |
Increasing the frequency of incident light
BUT
| Increasing the intensity of incident light
BUT
|
9.2.2 Calculating energy of photoelectrons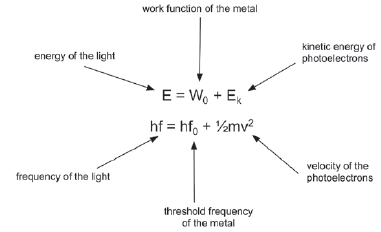
9.2.3 Conditions for emission of photoelectrons
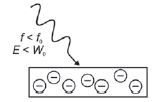
1. f light < fo
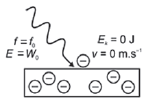
| 2. f light < fo
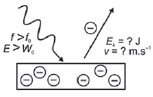
| 3. f light < fo
|
E: energy of light (J)
h: Planck’s constant (6,63 × 10–34 J·s–1)
f: frequency (Hz)
W0: work function (J)
Ek: kinetic energy (J)
v: velocity of electrons (m·s–1)
m: mass of electron (9,11 × 10−31 kg)
9.2.4 Another example of the photoelectric effect
A photoelectric diode in an electric circuit is another example of the application of the photoelectric effect. When photons (light) with a frequency higher than the cut-off frequency of the metal cathode shines on the cathode, photoelectrons are emitted.
Photoelectric diodes are used in:
- smoke detectors
- light meters in cameras
- remote controls and
- CD players.
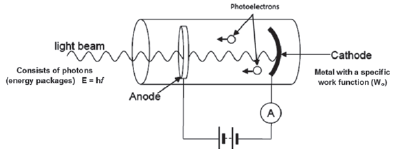
A photoelectric diode in an electric circuit
Worked example 1
Calculate the energy of a light wave with a wavelength of 660 nm.
Solutions
c = λf
∴ 3 × 108 = 660 × 10–9 f
f = 3 × 108 – 4,55 × 1014 Hz
E = hf
E = (6,63 × 10–34)(4,55 × 1014)
E = 3,02 × 10–19 J
When the wavelength is given, always calculate the frequency first.
Convert nm to m 660 nm = 660 × 10–9 m
Use c = λf
Then calculate the energy using E = hf.
Worked example 2
A learner wants to demonstrate the photoelectric effect.
He uses a disk of zinc placed on an electroscope.
The work function (W0) of zinc is 6,9 × 10–19 J.
- Define the concept work function.
- Calculate the maximum wavelength of light that will eject electrons from the zinc.
- The electroscope is negatively charged and then exposed to ultraviolet light from a mercury discharge lamp. One of the wavelengths of the light is 260 nm. Calculate the kinetic energy of an electron emitted from the zinc disk by a photon of this light.
Solutions
- The work function (W0) of a metal is the minimum energy that is required to emit a photoelectron form the surface of the metal.
- E = hf and c = λf
6,9 × 10–19 = (6,63 × 10–34) f 3 × 108 = λ (1,05 × 1015)
f = 1,04 × 1015 Hz λ = 2,88 × 10–7 m - E = W0 + Ek
E = hf
hf = W0+ Ek
(6,63 × 10–19)(1,15 × 1015) = 6,9 × 10–19 + Ek
Ek = (7,63 × 10–19) – (6,9 × 10–19)
Ek = 7,3 × 10–20 J
And
c = λf
3 × 108 = (260 × 10–9) f
f = 3 × 108 / (260 × 10–9)
f = 1,15 × 1015 Hz
Remember: Convert nm to m 260 nm = 260 × 10–9 m
Activity 1
A metal surface is illuminated with ultraviolet light of wavelength 330 nm. Electrons are emitted from the metal surface. The minimum amount of energy required to emit an electron from the surface of this metal is 3,5 × 10–19 J.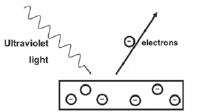
- Name the phenomenon illustrated. (1)
- Give ONE word or term for the underlined sentence in the above paragraph. (1)
- Calculate the frequency of the ultraviolet light. (3)
- Calculate the kinetic energy of a photoelectron emitted from the surface of the metal when the ultraviolet light shines on it. (3)
- The intensity of the ultraviolet light illuminating the metal is now increased.
What effect will this change have on the following?- Kinetic energy of the emitted photoelectrons. (Write down only INCREASES, DECREASES or REMAINS THE SAME.) (1)
- Number of photoelectrons emitted per second. (Write down only INCREASES, DECREASES or REMAINS THE SAME.) (1)
[10]
Solutions
|
Activity 2
In the simplified diagram below, light is incident on the emitter of a photocell. The emitted photoelectrons move towards the collector and the ammeter registers a reading.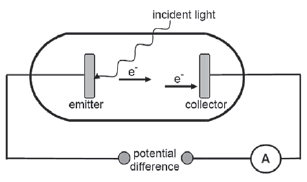
- Name the phenomenon illustrated above. (1)
- The work function of the metal used as emitter is 8,0 × 10–19 J. The incident light has a wavelength of 200 nm. Calculate the maximum speed at which an electron can be emitted. (6)
- Incident light of a higher frequency is now used.
How will this change affect the maximum kinetic energy of the electron emitted in the question above?
Write down only INCREASES, DECREASES or REMAINS THE SAME. (1) - The intensity of the incident light is now increased.
How will this change affect the speed of the electron calculated in QUESTION 11.1.2? Write down INCREASES, DECREASES or REMAINS THE SAME. Give a reason for the answer. (2) - A metal worker places two iron rods, A and B, in a furnace.
After a while he observes that A glows deep red while B glows orange. Which rod A or B has higher energy of radiation?
Give a reason for your answer. (2) - Neon signs illuminate many buildings.
What type of spectrum is produced by neon signs? (1)
[13]
Solutions
|
Activity 3
During an investigation, light of different frequencies is shone onto the metal cathode of a photocell. The kinetic energy of the emitted photoelectrons is measured. The graph below shows the results obtained.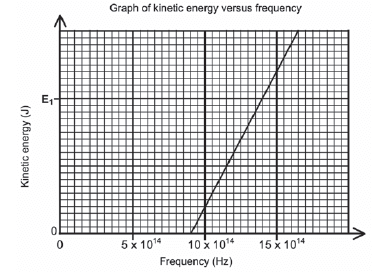
- For this investigation, write down the following:
- Dependent variable (1)
- Independent variable (1)
- Controlled variable (1)
- Define the term threshold frequency. (2)
- Use the graph to obtain the threshold frequency of the metal used as cathode in the photocell. (1)
- Calculate the kinetic energy at E1 shown on the graph. (4)
- How would the kinetic energy calculated in QUESTION 11.4 be affected if light of higher intensity is used? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
[11]
Solutions
|