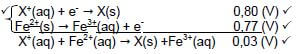PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - AMENDED SENIOR CERTIFICATE EXAMS PAST PAPERS AND MEMOS MAY/JUNE 2018
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
AMENDED SENIOR CERTIFICATE EXAMS
PAST PAPERS AND MEMOS
MAY/JUNE 2018
MEMORANDUM
QUESTION 1
1.1 D ✓✓ (2)
1.2 A ✓✓ (2)
1.3 B ✓✓ (2)
1.4 B ✓✓ (2)
1.5 D ✓✓ (2)
1.6 C ✓✓ (2)
1.7 B ✓✓ (2)
1.8 D ✓✓ (2)
1.9 D ✓✓ (2)
1.10 C ✓✓ (2) [20]
QUESTION 2
2.1
2.1.1 A ✓ (1)
2.1.2 D ✓ (1)
2.1.3 B ✓ (1)
2.1.4 E ✓ (1)
2.1.5 B ✓ (1)
2.2
2.2.1 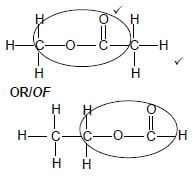
Marking criteria
|
Accept
- Any correct arrangement of correct number of atoms (2)
2.2.2 ANY ONE:
- Methyl ✓ethanoate ✓
OR/OF - Ethyl ✓methanoate ✓ (2)
2.3
2.3.1 A large molecule ✓composed of smaller monomer units covalently bonded to each other in a repeating pattern. ✓ (2)
2.3.2 Polyethene ✓
Accept:
- Polyethylene/polythene (1)
2.3.3 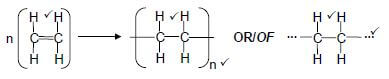
Accept as reactant: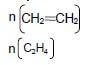
Accept as product:![]() (3)
(3)
2.4 Hydrolysis/Substitution ✓ (1)
Marking guidelines
|
2.5
- Use concentrated strong base/NaOH/KOH/LiOH OR ethanolic/alcoholic strong base/NaOH/KOH/LiOH. ✓/Use ethanol instead of water./No water.
- Heat strongly ✓
Accept: Increase temperature (2) [18]
QUESTION 3
3.1
- Structure:
The chain length/molecular size /molecular structure/molecular mass/ surface area increases. ✓ - Intermolecular forces:
Increase in strength of intermolecular forces/induced dipole /London/ dispersion /Van der Waals forces/momentary dipoles. ✓ - Energy:
More energy needed to overcome/break intermolecular forces. ✓
OR
Structure:
From 4 C atoms to 1 C atom/bottom to top the chain length/molecular size/molecular structure/molecular mass/surface area decreases. ✓ - Intermolecular forces:
Decrease in strength of intermolecular forces/ induced dipole forces/ London forces/dispersion forces. ✓
Energy:
Less energy needed to overcome/break intermolecular forces. ✓ (3)
3.2
- Alkanes have London/dispersion/induced dipole forces. ✓
- Alcohols have hydrogen bonding (in addition to London/dispersion/ induced dipole forces and dipole dipole forces). ✓
- Hydrogen bonding are stronger intermolecular forces than London/ dispersion/ induced dipole forces. ✓
OR/OF
More energy needed to overcome/break intermolecular forces in alcohols - Alcohols have higher boiling points than alkanes. ✓ (4)
3.3 Decrease ✓ (1)
3.4 Lower than✓ - 2-methylpropane/It is more branched/has a smaller surface area/has a shorter chain length (than butane/chain isomer) ✓
OR
Butane/chain isomer is less branched /has larger surface area/longer chain length (than 2-methylpropane). (2) [10]
QUESTION 4
4.1
4.1.1 Substitution/halogenation/bromonation✓ (1)
4.1.2 Elimination/dehydration ✓ (1)
4.1.3 Esterification/condensation ✓ (1)
4.1.4 Addition/hydrohalogenation/hydrobromonation ✓ (1)
4.2
4.2.1 Catalyst/dehydrating agent/speeds up reaction ✓ (1)
4.2.2 Propyl ✓ ethanoate ✓/Propieletanoaat (2)
4.2.3 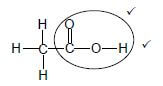 (2)
(2)
Marking criteria:
| |
IF:
|
4.3 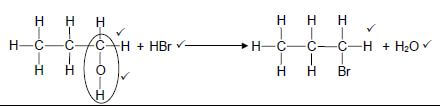 (5)
(5)
Notes:
|
[14]
QUESTION 5
5.1 ONLY ANY ONE OF:
- Change in concentration of products/reactants ✓ per (unit) time. ✓
- Rate of change in concentration. ✓✓
- Change in amount/number of moles/volume/mass ✓ of products or reactants per (unit) time. ✓
- Amount/number of moles/volume/mass (of products) formed/(reactants) used✓ per (unit) time.✓ (2)
5.2
5.2.1 Surface area/State of division ✓ (1)
5.2.2 ANY ONE:
- Amount/mass of magnesium ✓
- Concentration of HCℓ/acid
- (Initial) temperature (1)
5.3
5.3.1 (5)
Marking criteria:
| |
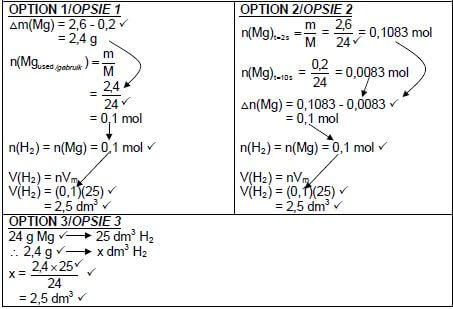 |
5.3.2
Marking criteria
|
ave rate / tempo = ∆n ✓
∆t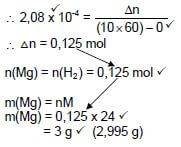 (5)
(5)
5.4
- Larger surface area/state of division. ✓
- More particles (per volume) with correct orientation ✓
OR - More contact points./Meer kontakpunte.
- More effective collisions per (unit) time./Frequency of effective collisions increases./More particles collide with sufficient kinetic energy & correct orientation per (unit) time.✓✓ (3) [17]
QUESTION 6
6.1 The stage in a chemical reaction when the rate of forward reaction equals the rate of reverse reaction./Both forward and reverse reactions take place at same rate. ✓✓
OR
The stage in a chemical reaction when the concentrations of reactants and products remain constant. ✓✓ (2)
6.2
6.2.1 2 ✓ (1)
6.2.2 1 ✓ (1)
6.2.3 3 ✓ (1)
6.3 POSITIVE MARKING FROM QUESTION 6.2.
Marking criteria:
|
OPTION 1
|
OPTION 2
Kc = [C]3 = 6,75 ✔ (7)
|
USING CONCENTRATION
Kc = [C]3 = 6,75 ✔ (7)
|
6.4 Endothermic ✔
- (An increase in temperature) favours the reverse reaction. ✔
- An increase in temperature favours an endothermic reaction. ✔ (3) [15]
QUESTION 7
7.1 Titration/Volumetric analysis ✓ (1)
7.2 To measure the (exact) volume of acid needed to reach endpoint/to neutralise the base. ✓ (1)
7.3 Acids produce hydrogen ions (H+)/hydronium ions (H3O+) in solution/when dissolved in water. ✓✓
IF:
- Acids produce hydrogen ions (H+)/hydronium ions (H3O+). ✓ (2)
7.4 H2SO4 ionises completely.✓ (1)
7.5 Blue to yellow✓ (1)
7.6 (4)
Marking guidelines:
| |
OPTION 1 | OPTION 2 |
7.7 POSITIVE MARKING FROM QUESTION 7.6.
Marking guidelines:
| |
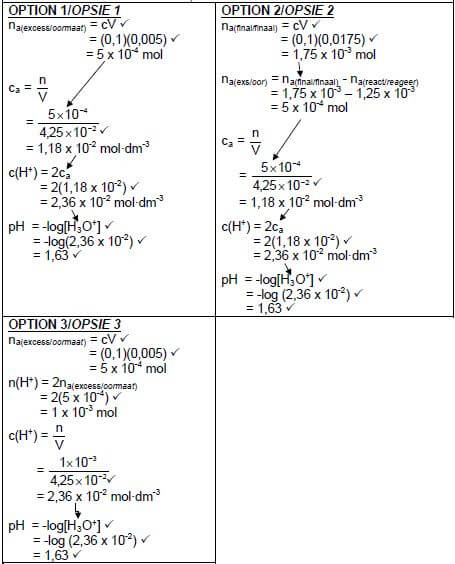 (7) (7) |
[17]
QUESTION 8
8.1
8.1.1 Galvanic (cell)/Voltaic (cell) ✓ (1)
8.1.2 Indicates phase boundary./Interphase /phase separator✓ (1)
8.1.3 Fe2+ → Fe3+ + e- ✓✓ (2)
Notes
|
8.1.4 (5)
OPTION 1 | Notes
|
OPTION 2 | |
8.2
8.2.1 Pt ✓ (1)
8.2.2 Iron(III) (ions)Ferric ions✓ (1)
8.2.3 2Fe3+ + Cu ✓→ 2Fe2+ + Cu2+ ✓ Bal. ✓ (3)
Notes
|
[14]
QUESTION 9
9.1
9.1.1 Electrolyte ✓ (1)
9.1.2 Conduct electricity/Carry charges ✓ (1)
9.2 Cu(NO3)2 ✓ (1)
9.3 Iron rod✓ - Reduction takes place. ✓ (2)
9.4 Cu → Cu2+ + 2e-✓✓ (2)
Notes
|
9.5
9.5.1 Copper(II) (ions)/Cu2+ ✓and silver (ions)/Ag+ ✓
Accept
- Cu (ions) and Ag (ions) (Ions are stated in the question.) (2)
9.5.2 Ag+/silver(I) ions is a stronger oxidising agent ✓ than Cu2+/Copper(II) ions and will be reduced (more readily) ✓ to form silver/Ag on the iron rod. (2 ) [11]
QUESTION 10
10.1
10.1.1 (Catalytic) oxidation (of ammonia)✓ (1)
10.1.2 Neutralisation/acid-base reaction ✓ (1)
10.2
10.2.1 Nitrogen/N2✓ (1)
10.2.2 NO2/nitrogen dioxide✓ (1)
10.2.3 Nitric acid/HNO3✓ (1)
10.3
10.3.1 2NH3 + H2SO4 ✓ → (NH4)2SO4 ✓ Bal. ✓ (3)
Notes:
|
10.3.2 4NH3 + 5O2 ✓ → 4NO + 6H2O ✓ Bal. ✓ (3)
Notes :
|
10.4 % N = 28 × 100 ✓
80 ✓
= 35% ✓ (3) [14]
TOTAL: 150