TECHNICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - 2018 JUNE EXAM PAST PAPERS AND MEMOS
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE
MEMORANDUM
JUNE 2018
QUESTION 1
1.1 B ✓✓ (2)
1.2 C ✓✓ (2)
1.3 B ✓✓ (2)
1.4 D ✓✓ (2)
1.5 D ✓✓ (2)
1.6 C ✓✓ (2)
1.7 C ✓✓ (2)
1.8 A ✓✓ (2)
1.9 B ✓✓ (2)
1.10 C✓✓ (2)
[20]
QUESTION 2
2.1.1 B ✓ (1)
2.1.2 A ✓ (1)
2.1.3 E ✓ (1)
2.1.4 B ✓
C ✓ (2)
2.1.5 D ✓ (1)
2.2.1 Alkane ✓ (1)
2.2.2 Ester OR Carboxylic acid (1)
2.3.1 Butan-2-one / 2-butanone / butanone ✓✓(2)
2.3.2 4-ethyl-5-methylhex-2-yne / 4-ethyl-5-methly-2-hexyne
Marking criteria:
- Stem (hexyne)✓
- Two methyl groups and one ethyl group✓
- Correct numbering of substituents and functional group✓
IF:
Any error e.g. hyphens omitted and/or incorrect sequence: Max. ¾(3)
2.4.1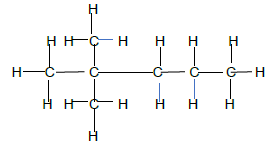
| Marking Criteria Whole structure correct: 2/2 5 Carbon atoms in longest chain chain ½ |
(2)
2.4.2 CH3CH2CH2CH2OH ✓ (1)
2.5.1 Compounds with the same molecular formula ✓ but different structural formula ✓(2)
2.5.2 Esterification ✓(1)
2.5.3 Catalyst /Speeds up reaction/Dehydrating agent ✓ (1)
2.5.4 methanoic acid ✓✓ (2)
2.5.5 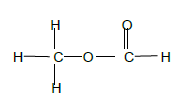
Methyl✓ methanoate ✓
| Marking Criteria Whole structure: 2/2 Only functional group correct : ½ |
(4)
[26]
QUESTION 3
3.1.1 Measure of resistance to flow (2)
3.1.2 (Contains) single bonds only ✓✓ (2)
3.1.3 Chain length√/Surface area/Molecular size (Any one) ✓ (1)
3.1.4 The longer the (carbon) chain✓ the higher the viscosity✓ OR
The shorter the chain the lower the viscosity OR
The longer the chain the lower the viscosity OR
The shorter the chain the higher the viscosity (2)
3.1.5 From A to C
- Chain length/Surface area/Molecular size increases ✓
- Strength of intermolecular forces /London/induced dipole forces/dispersion forces increases ✓
- More energy needed to overcome intermolecular forces ✓ (3)
OR
From C to A
- Chain length/Surface area/Molecular size decreases ✓
- Strength of intermolecular forces /London/induced dipole forces/dispersion forces decreases ✓
- Less energy needed to overcome intermolecular forces ✓
3.1.6 C ✓
Highest viscosity✓ (2)
3.1.7 2C6H14 + 19O2 → 12CO2 + 14H2O✓
Balancing ✓ (3)
3.2.1 Thermometer ✓(1)
3.2.2 The longer the (carbon) chain the higher the boiling point ✓
OR The shorter the (carbon) chain the lower the boiling point (2)
3.2.3 -42 and -0,5 ✓ (oC) (1)
3.2.4 Position of -OH✓✓ /hydroxyl group is the same (at position 1) (2)
3.3 Alcohols have (London forces, dipole-dipole forces ) hydrogen bonds ✓
Alkanes have London forces
Hydrogen bonds are stronger than London forces ✓ (3)
[24]
QUESTION 4
4.1.1 Addition /Hydrogenation ✓ (1)
4.1.2 Hydration ✓ (1)
4.1.3 Substitution/Hydrolysis ✓ (1)
4.1.4 C2H6 ✓ (1)
4.1.5 Platinum ✓ (1)
4.1.6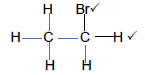
| Marking Criteria Whole structure: 2/2 Only functional group correct : ½ |
(2)
4.1.7 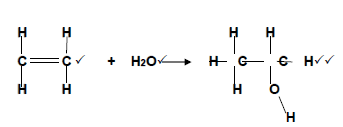
(4)
4.1.8 KOH or NaOH ✓ (1)
4.1.9 Br2 / Bromine ✓ (1)
4.1.10 Sunlight / (mild) Heat ✓ (1)
4.2.1 Molecule containing large number of covalently bonded monomer units ✓✓(2)
4.2.2 Make plastic containers /Electrical insulation (Any correct answer) ✓ (1)
4.2.3 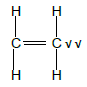
Ethane ✓
| Marking Criteria Whole structure: 2/2 Only functional group correct : ½ |
(3)
4.2.4 Addition ✓ (1)
[21]
QUESTION 5
5.1.1 Angle of incidence ✓ (1)
5.1.2 Angle of emergence ✓ (1)
5.2 35o ✓ (1)
5.3.1 PQ incident ray ✓ (1)
5.3.2 QR refracted ray ✓ (1)
5.3.3 RS emergent ray ✓ (1)
5.4.1 B ✓
5.4.2 Refracted ray towards normal ✓✓ (3)
[9]
QUESTION 6
6.1.1 (Phenomenon whereby) light breaks up into its component colours ✓✓ (2)
6.1.2
- Violet ✓ (1)
- Red ✓ (1)
6.1.3 DECREASES ✓
Frequency is constant ✓ v =f λ Therefore v ά λ (3)
6.2.1 Accelerating charge ✓✓ (2)
6.2.2 Red ✓ (1)
6.2.3
- X-rays ✓ (1)
- Radio waves ✓ (1)
- UV ✓ (1)
6.2.4
- Radio waves✓ X-rays✓ Infra-red✓(3)
- Radio waves Infra-red X-rays ✓✓ (Correct order) (2)
6.2.5 Quantum of energy ✓✓ (2)
6.2.6
- v =f λ √
3 x 108√ = f (400 x 10 -9)
7,5 x 1014 Hz = f √ (3) - v =f λ
3 x 108√ = f ( 10-2 x 10-9) √
3 x 1019 Hz= f
E = hf√
= 6,63 X 10 -34 x 3 x 1020√
= 1,99 x 10-14 J√ (4)
[27]
QUESTION 7
7.1 Reflection when light bounces off a surface ✓✓(2)
7.2 Angle of incidence = angle of reflection ✓
The incident ray, reflected ray and normal lie in the same plane ✓ (2)
7.3.1 SAME SIZE ✓ (1)
7.3.2 12 cm ✓ (1)
7.3.3 VIRTUAL ✓ (1)
7.4.1 Total internal reflection ✓(1)
7.4.2 Light must travel from a denser to a less dense medium✓
Angle of incidence greater than critical angle ✓(2)
7.4.3 Endoscope ✓ (1)
[11]
QUESTION 8
8.1 CONVEX ✓
Light rays converge ✓ (2)
8.2.1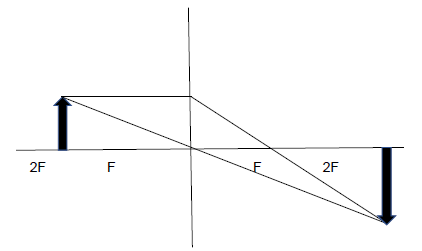
Marking criteria
|
(6)
8.2.2 The image is always smaller than the object (for a concave lens) ✓✓ (2)
8.3.1 Camera ✓ (1)
8.3.2 Projector ✓(1)
[12]
TOTAL: 150