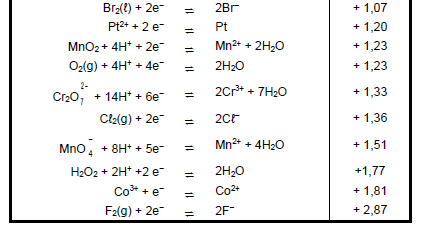PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - 2018 JUNE EXAM PAST PAPERS AND MEMOS
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE
JUNE 2018
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of SEVEN questions. Answer ALL the questions in the ANSWERBOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number your answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub-questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four possible options are provided as answers to the following questions. Each question has only ONE correct answer. Write only the correct letter (A–D) next to the question number (1.1–1.10) in the ANSWERBOOK for example 1.11 E.
1.1 Which ONE of the following statements is CORRECT about methane?
Compared to members of its homologous series, methane has the …
- highest boiling point.
- Highest melting point.
- lowest vapour pressure.
- highest vapour pressure. (2)
1.2 Which ONE of the following compounds is an alcohol?
| A | O ║ CH3CH2C — H | B | O ║ CH3CCH3 |
| C | OH ║ CH3CHCH3 | D | CH3COOCH3 |
(2)
1.3 The condensed structural formula of a polymer, P is given below.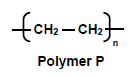
The IUPAC name of the MONOMER of polymer P is …
- ethane.
- ethene.
- ethyne.
- polythene. (2)
1.4 Which ONE of the following statements is TRUE about a reaction at equilibrium?
At equilibrium the concentration of products …
- is equal to the concentration of reactants.
- is always greater than that of reactants.
- is always smaller than that of reactants.
- remains constant over a period of time. (2)
1.5 ONE mole of zinc reacts with EXCESS hydrochloric acid of concentration 1 mol.dm-3 at 25 °C according to the balanced equation.
Zn(s) + 2HCℓ(aq) → ZnCℓ2(aq) + H2(g)
Which ONE of the following changes will increase the average kinetic energy of reacting particles?
- Add more zinc
- Increase temperature
- Add a suitable catalyst
- Increase concentration of HCℓ (2)
1.6 The following reaction reaches equilibrium in a closed container.
H2(g) + I2(g) ⇌ 2HI(g) ΔH > 0
What effect will a decrease in temperature have on the number of moles of HI at equilibrium and the Kc value?
| Number of moles | Kc value | |
| A | Increases | Increases |
| B | Decreases | Increases |
| C | Increases | Decreases |
| D | Decreases | Decreases |
(2)
1.7 Which ONE of the following is a product in ALL neutralisation reactions?
- H2O
- Acid
- Base
- NaCℓ (2)
1.8 An acid solution has a concentration of 0,1 mol.dm-3. The concentration of hydronium ions, [H3O+], in the solution is found to be 0,00009 mol.dm-3.
The conclusion that can be drawn from above information is that the acid is …
- weak.
- strong.
- diprotic.
- concentrated. (2)
1.9 Which ONE of the following solutions, each of concentration 0,1 mol.dm-3 has the lowest pH?
- KOH
- NH4Cℓ
- Na2CO3
- CH3COONa (2)
1.10 Consider the potential energy diagram for the reaction represented by the balanced equation given below.
2CO2(g) ⇌ 2CO(g) + O2(g)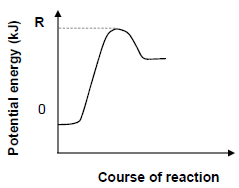
What does energy R represent?
R represents the …
- heat of reaction for the reverse reaction.
- heat of reaction for the forward reaction.
- activation energy for the reverse reaction.
- activation energy for the forward reaction. (2)
[20]
QUESTION 2 (Start on a new page.)
Study the compounds given below and answer the questions that follow.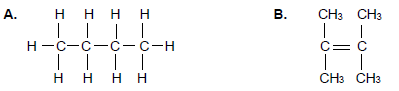
2.1.1 Both compounds A and B are hydrocarbons.
Define the term hydrocarbon. (2)
For compound A write down:
2.1.2 The NAME of the homologous series to which it belongs (1)
2.1.3 The EMPIRICAL formula (1)
2.1.4 A balanced equation for the reaction between compound A and excess oxygen using MOLECULAR FORMULAE (3)
2.1.5 Is the reaction mentioned in QUESTION 2.1.4 above EXOTHERMIC or ENDOTHERMIC? (1)
Compound A can undergo the cracking process according to the incomplete equation shown below.
2.1.6 Define the term cracking. (2)
2.1.7 Write down the STRUCTURAL FORMULA of the alkene. (2)
2.1.8 Give the NAME of the type of cracking used in the reaction above. (1)
For compound B answer the following questions.
2.1.9 Classify compound B as SATURATED or UNSATURATED.
Give a reason for the answer. (2)
2.1.10 Write down the IUPAC name of compound B. (3)
2.2 Consider the compounds, C and D given below.
C. 5-ethyl-4-methylhept-2-yne
D. O
║
CH3CCH2CH2CH3
Write down the:
2.2.1 STRUCTURAL FORMULA of compound C (3)
2.2.2 IUPAC name of compound D (2)
[23]
QUESTION 3 (Start on new page.)
The boiling points of some organic compounds are given in the table below. X represents an unknown boiling point. Compounds A and B have different functional groups.
| COMPOUND | FORMULA | BOILING POINTS (°C) |
| A | O ║ CH3CH2CH2CH2 C — H | 103 |
| B | O ║ CH3CH2CH2CH2 C — OH | X |
| C | CH3CH2CH2CH2CH3 | 36,1 |
| D | CH3 Ι CH3 — C —CH3 Ι CH3 | 9,5 |
3.1 Define the term functional group. (2)
3.2 Write down the NAME of the:
3.2.1 HOMOLOGOUS SERIES to which compound A belongs (1)
3.2.2 FUNCTIONAL GROUP of compound B (1)
3.3 Consider the boiling points given below.
| 8,1 °C | 95 °C | 185,4 °C |
3.3.1 From these boiling points, choose the boiling point represented by X in the table above. (1)
3.3.2 Fully explain how you arrived at the answer to QUESTION 3.3.1. (3)
3.4 Compounds C and D are structural isomers.
3.4.1 Define the term structural isomer. (2)
3.4.2 What TYPE of structural isomers are compounds C and D?
Choose from FUNCTIONAL, POSITIONAL or CHAIN. (1)
3.4.3 How does the vapour pressure of compound C compare to that of D?
Choose from SMALLER THAN, HIGHER THAN or EQUAL TO.
Explain the answer fully. (4)
[15]
QUESTION 4 (Start on a new page.)
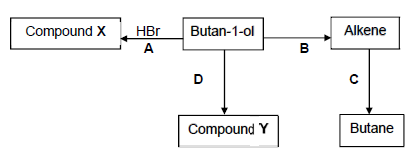
The flow diagram shows how an alcohol can be used to prepare other organic compounds. The letters A, B, C and D represent different organic reactions. X and Y are organic compounds.
4.1 Write down the type of reaction represented by:
4.1.1 A (1)
4.1.2 B (1)
4.2 For reaction C write down the:
4.2.1 TYPE OF ADDITION reaction (1)
4.2.2 NAME or FORMULA of the catalyst used (1)
4.3 Using STRUCTURAL FORMULAE, write down a balanced equation for reaction A. (4)
4.4 For reaction B write down the:
4.4.1 NAME or FORMULA of the inorganic reactant used (1)
4.4.2 STRUCTURAL FORMULA of the alkene produced (2)
4.5 Reaction D is an esterification reaction. Compound Y is a FUNCTIONAL isomer of pentanoic acid.
For reaction D write down the:
4.5.1 TWO reaction conditions needed (2)
4.5.2 STRUCTURAL FORMULA of the carboxylic acid used (2)
4.5.3 IUPAC name of compound Y (2)
[17]
QUESTION 5 (Start on a new page.)
5.1 Study the reaction given below in which EXCESS magnesium ribbon (Mg) reacts with 50 cm3 of a diluted sulphuric acid (H2SO4) solution at room temperature.
Mg(ribbon) + H2SO4(aq) → MgSO4(aq) + H2(g) ΔH < 0
What changes can be made to the following substances to increase the rate of reaction?
5.1.1 Magnesium (1)
5.1.2 H2SO4 (1)
How will EACH of the following changes affect the reaction rate?
Choose from DECREASES, INCREASES or NO EFFECT
5.1.3 Decrease in temperature (1)
5.1.4 Increase in pressure (1)
5.2 A group of learners uses the reaction between zinc and EXCESS diluted hydrochloric acid (HCℓ) to investigate one of the factors that affects the rate of a chemical reaction. The balanced equation for the reaction is:
Zn(s) + 2HCℓ(aq) → ZnCℓ2(aq) + H2(g)
The same volume of HCℓ and same amount of Zn was used.
| Experiment | T (°C) | Volume HCℓ (cm3) | Concentration of HCℓ (mol.dm-3) | State of division of Zn |
| I | 30 | 100 | 0,40 | Powder |
| II | 30 | 100 | 0,40 | Granules |
5.2.1 Write down the independent variable for the investigation. (1)
The results obtained for experiment I and II are shown in the graph below.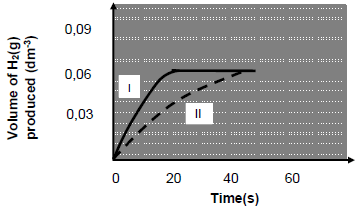
5.2.2 Explain why the graph for experiment I becomes horizontal after 20 seconds. (2)
5.2.3 How does the amount of zinc used in experiment I compare to the amount of zinc used in experiment II.
Choose from HIGHER THAN, LOWER THAN or EQUAL TO.
Give a reason for the answer. (2)
5.2.4 Calculate the:
- Average rate of reaction (in dm3.s-1) from time 0 to 30 seconds for experiment I (3)
- Initial number of moles of hydrochloric acid in experiment II (3)
- Mass of hydrochloric acid that remains at the completion of the reaction in experiment II
Take the MOLAR VOLUME of gas as 24,3 dm3.mol-1 at 25 °C. (5)
The learners conduct TWO more experiments (experiment III and IV) in order to investigate another factor that affects rate of reaction.
The reaction conditions are summarised in the table below.
| Experiment | T (°C) | Volume HCℓ (cm3) | Concentration of HCℓ (mol.dm-3) | State of division of Zn |
| III | 30 | 100 | 0,40 | Powder |
| IV | 30 | 100 | 0,40 | Powder |
The diagram below represents the Maxwell-Boltzman distribution curve for the reactions in experiment III and IV.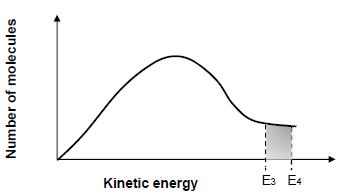
E3 is the activation energy for the reaction in experiment III and E4 is the activation energy for the reaction in experiment IV.
5.2.5 Define activation energy. (2)
5.2.6 In which experiment (experiment III or experiment IV) is the reaction rate HIGHER? (1)
5.2.7 Explain how the factor responsible for the difference in the reaction rate of experiment III and experiment IV affects reaction rate by referring to the collision theory. (4)
[27]
QUESTION 6 (Start on a new page.)
Sulphur (S) is allowed to react with hydrogen gas (H2) in a closed container according to the balanced equation:
H2(g) + S(s) ⇌ H2S(g) ΔH < 0
The reaction reaches chemical equilibrium after some time.
6.1 Define chemical equilibrium. (2)
6.2 Write down TWO conditions that make it possible for the reaction to reach equilibrium. (2)
6.3 What effect will the following changes have on the amount of H2S at equilibrium?
Choose from INCREASES, DECREASES or NO EFFECT.
6.3.1 Adding more sulphur. (1)
6.3.2 Adding more H2. (1)
6.4 The graph below shows the changes in the rate of reaction from the moment the reactants are introduced in the container.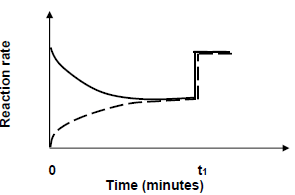
6.4.1 Which reaction is represented by the dotted line?
Choose from FORWARD or REVERSE. (1)
6.4.2 State TWO possible changes that could be made to the reaction conditions at t1. (2) Reaction rate
6.5 The reaction is started by heating a mixture of hydrogen gas (H2) and excess sulphur (S) in a closed container of volume V. The reaction establishes equilibrium at 210 °C.
At equilibrium 17g of H2S are present.
The value of the equilibrium constant, Kc is 2,56 x 10-1 at 210 °C.
6.5.1 Is there a HIGH or LOW yield at 210 °C? Give a reason for the answer. (2)
Calculate the:
6.5.2 Initial number of moles of hydrogen (H2) placed in the container (8)
6.5.3 Initial number of moles of sulphur (S) if only 90% of the original amount of sulphur is present at equilibrium. (3)
Three graphs of Kc versus temperature are shown below.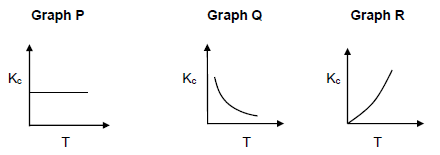
6.5.4 Which ONE of the graphs can possibly be the Kc versus temperature graph for the reaction?
Explain the answer fully. (4)
[26]
QUESTION 7 (Start on a new page.)
7.1 An ion of a salt reacts with water according to the following balanced equation.
CO32-(aq) + H2O(ℓ) ⇌ HCO3-(aq) + OH-(aq)
7.1.1 Write down a term for the underlined phrase. (1)
7.1.2 Give a reason why the reaction shown above is said to be an acidbase reaction according to the Lowry-Bronsted model. (1)
7.1.3 Write down the FORMULA of the conjugate base of water. (1)
7.1.4 The carbonate ion CO32- is a weak base.
Which ONE of the following values is a possible value for the dissociation constant, Kb at 25 °C for the carbonate ion?
| Kb < 1 x10-14 | Kb > 1 x10-14 | Kb = 1 x10-14 |
(1)
The hydrogen carbonate ion, HCO3- can act as an ampholyte.
7.1.5 Define the term ampholyte. (2)
For the hydrogen carbonate ion HCO3- ion write down the formula of the:
7.1.6 Conjugate acid (1)
7.1.7 Conjugate base (1)
7.2 A solution of a strong diprotic acid has pH = 1,3.
7.2.1 Define the term diprotic acid. (2)
7.2.2 Calculate the concentration of the hydroxide ions in the solution. (4)
7.3 The diprotic acid mentioned in QUESTION 7.2 are diluted by adding 8 cm3 of the acid to water to make 100 cm3 of solution.
During a titration the 25 cm3 dilute acid neutralises exactly 14,2 cm3 of sodium hydroxide solution.
7.3.1 Calculate the concentration of the sodium hydroxide solution. (6)
In the reaction the ratio of BASE : ACID is 2 : 1.
Three indicators A, B and C available for the titration are shown in the diagram below.
| INDICATOR | pH range |
| A | 2,0–4,3 |
| B | 6,8–7,4 |
| C | 8,6–10,2 |
7.3.2 Which indicator must be used in the titration?
Give a reason for the answer. (2)
[22]
TOTAL: 150
NATIONAL SENIOR CERTIFICATE
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
TABLE 4A: STANDARD REDUCTION POTENTIALS