PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - 2018 JUNE EXAM PAST PAPERS AND MEMOS
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES PAPER 2
GRADE 12
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
JUNE 2018
QUESTION 1
1.1 D ✓✓ (2)
1.2 C ✓✓ (2)
1.3 B ✓✓ (2)
1.4 D ✓✓ (2)
1.5 B ✓✓ (2)
1.6 D ✓✓ (2)
1.7 A ✓✓ (2)
1.8 A ✓✓ (2)
1.9 B ✓✓ (2)
1.10 D ✓✓ (2)
[20]
QUESTION 2
2.1.1 Organic compound that consist of carbon and hydrogen atoms only. ✓✓ (2 or/of 0) (2)
2.1.2 Alkanes ✓(1)
2.1.3 C2H5 ✓ (1)
2.1.4 2C4H10 + 13O2 ✓ → 8CO2 + 10H2O ✓ Bal ✓
Notes:
|
2.1.5 EXOTHERMIC ✓ (1)
2.1.6 The chemical process in which longer chain hydrocarbon molecules are broken✓ into shorter more useful molecules. ✓(2)
2.1.7
Marking criteria:
|
2.1.8 CATALYTIC✓ (1)
2.1.9 UNSATURATED✓
Contains double bonds OR multiple bonds between C atoms.✓ (2)
2.1.10 2,3-dimethylbut-2-ene/2,3-dimethyl-2-buteen
Marking Criteria:
|
2.2.1
2.2.2 Pentan-2-one✓✓/2-pentanone (2)
[23]
QUESTION 3
3.1 A bond or an atom or a group of atoms that determine(s) the physical and chemical properties of a group of organic compounds. ✓✓ (2 or/of 0) (2)
3.2.1 Aldehyde ✓(1)
3.2.2 Carboxile group ✓(1)
3.3.1 185,4(°C) ✓ (1)
3.3.2
- Compound B/carboxylic acid has hydrogen bonding ✓(in addition to London forces/Dispersion forces/Induced dipole forces/dipole-dipole forces.).
- Hydrogen bonds are stronger ✓ than London forces/Dispersion forces/Induced dipole forces and dipole-dipole forces.
- More energy will be needed to overcome/break(intermolecular) forces. ✓ (3)
3.4.1 Organic molecules with the same molecular formula ✓ but different structural formulae. ✓(2)
3.4.2 CHAIN✓ (1)
3.4.3 SMALLER THAN ✓
STRUCTURE:
Compound D are branched/more compact/more spherical/smaller contact area/smaller surface(over which intermolecular forces act.) ✓
INTERMOLECULAR FORCES
Weaker/Less strength/Decrease in strength of Van der Waals forces/ London forces/Dispersion forces. ✓
ENERGY
Less energy needed to overcome/break (intermolecular) forces. ✓
OR
STRUCTURE:
Compound C is a straight chain/less compact/less spherical/larger contact area/larger surface(over which intermolecular forces act.) ✓
INTERMOLECULAR FORCES
Stronger/More strength/Increase in strength of Van der Waals forces/ London forces/Dispersion forces. ✓
ENERGY
More energy needed to overcome/break (intermolecular) forces. ✓(4)
[15]
QUESTION 4
4.1.1 Substitution ✓(1)
4.1.2 Eliminasie ✓/Dehydration(1)
4.2.1 Hydrogenation ✓(1)
4.2.2 Pt ✓/Ni/Pd/Platinum/Nickel/Palladium (1)
4.3
Notes:
|
4.4.1 (Concentrated) sulphuric acid ✓/hydrogen sulphate/H2SO4(1)
4.4.2
Marking criteria:
|
(2)
4.5.1
- Heat ✓/mild temperature over waterbath.
- Add concentrated sulphuric acid/H2SO4 ✓
(2)
4.5.2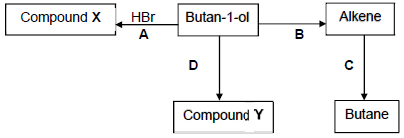
Marking criteria:
|
(2)
4.5.3 Butyl ✓ methanoate ✓(2)
[17]
QUESTION 5
5.1.1 Use magnesium powder. ✓ (1)
5.1.2 Increase concentration (H2SO4) ✓(1)
5.1.3 DECREASES ✓ (1)
5.1.4 NO EFFECT ✓(1)
5.2.1 Surface area ✓ (of Zn)/State of division(of Zn) (1)
5.2.2 Reaction stops ✓/come to completion/no more hydrogen gas is produced since zinc is used up. ✓ (2)
5.2.3 EQUAL TO ✓
The same Volume of H2(g) was produced. ✓ (2)
5.2.4
- Average rate/Gemiddelde tempo = ΔV/Δt
= (0,06 - 0) ✓/(30 - 0) ✓
= 2 x 10-3✓(mol.dm-3.s-1) or/of 0,002 (3) - n = cV ✓= 0,4 x 100/1000 ✓ = 0,04 mol ✓ (3)
Marking criteria:
- Use 24,3 dm3.mol-1 substituted in the correct formula.✓
- Calculate n(HCl) reacted using the mol ratio 1 : 2. ✓
- Calculate n(HCl) reacted ✓
- Use 36,5 g.mol-1 substitute in correct formule. ✓
- Finale antwoord/Final answer. (1,28–1,31 g)✓
POSITIVE MARKING from QUESTION 5.2.4 b
n(H2)produced = V/Vm= 0,06/24,3✓ = 2,47 x 10-3mol (0,002)
n( HCℓ)lreacted = 2 x 2,47 x 10-3✓ Ratio(0,004)
= 4,94 x 10-3 mol
n(HCℓ )remaining = ninitial - nproduced
= 0,04 – 4,94 x10-3✓
= 0,03506 mol (0,036)
m = nM = 0,03506 x 36,5✓= 1,28 g✓ Range (1,28–1,314 g) (5)
5.2.5 The minimum energy required to start a chemical reaction. ✓✓
(2 or 0) (2)
5.2.6 Experiment III ✓ (1)
5.2.7
- A catalyst ✓ provides an alternative pathway of lower activation energy(EA). ✓
- More particles will have sufficient/enough kinetic energy(Ek) to react. ✓ /More particles with Ek ≥ EA.
- More effective collisions per unit time/second. ✓
OR
Rate/frequency of effective collisions increases. (4)
[27]
QUESTION 6
6.1 Stage at which the rate of forward reaction equals the rate of reverse. ✓✓
OR
The stage where the concentrations/quantities of reactants and products remain constant. (2 or 0) (2)
6.2 Closed system ✓
Reversible reaction ✓(2)
6.3.1 NO EFFECT ✓ (1)
6.3.2 INCREASES ✓(1)
6.4.1 REVERSE ✓(1)
6.4.2 Increase in pressure ✓ /Decrease volume; Addition of a catalyst. ✓ (2)
6.5.1 LOW (yield) ✓
Kc is low. ✓ (2)
6.5.2
Marking Criteria:
|
OPTION 1
n(H2S)equilibrium = m/M = 17/34 ✓= 0,5 mol
| H2 | S | H2S | ratio✓ | |
| ninitial (mol) | 0 | |||
| Δn (mol) | 0,5 | 0,5 | 0,5 | |
| nequilibrium(mol) | 0,5 | |||
| cequilibrium (mol.dm-3) | nequilibrium/V | 0,5/V | ÷V ✓ |
Kc = [H2S]/[H2] ✓
2,56 x 10-1 ✓ = (0,5/V)/(n(H2)equilibrium/V) ✓
n(H2)equilibrium = 1,95 mol ✓
n(H2)initial = 0,5 + 1,95 = 2.45 mol ✓
(8)
6.5.3 POSITIVE MARKING from QUESTION 6.5.2
90/100 ni(S) ✓= ni(S) – 0,5 ✓
ni(S) = 5 mol ✓
(3)
6.5.4 Graph Q✓
- As temperature increases, Kc decreases. ✓
- [H2S] decreases✓/[H2] increases.
- Reverse reaction is favoured by an increase in temperature. ✓
(4)
[26]
QUESTION 7
7.1.1 Hydrolysis ✓(1)
7.1.2 Transfer of proton✓(H+) occurs./ CO32- gains a proton / H2O loses a proton.(1)
7.1.3 OH-✓ (1)
7.1.4 Kb < 1 x 10 -14 ✓ (1)
7.1.5 Substance that can act either as an acid or base./ ✓✓ (2)
7.1.6 H2CO3 ✓ (1)
7.1.7 CO3-2 ✓ (1)
7.2.1 An acid that donates TWO protons/H+/H3O+-ions. ✓✓(2)
7.2.2
pH = - log [H3O+]✓
1,3✓= - log [H3O+]
[H3O+]= 10-1,3
[OH-][H3O+] = 1 x 10-14
[OH-] x 10-1,3 =1 x 10-14✓
[OH-] = 10-12,7 mol.dm-3✓ = 1,995 x 10-13 mol.dm-3
(4)
7.3.1 POSITIVE MARKING FROM Q 7.2.2
OPTION 1
[H3O+] = 10-1,3 mol.dm-3
[Acid] = ½ x10-1,3 ✓ = 0,0251 mol.dm-3
Dilution c1V1= c2V2
0,0251(8) = c2 x 100✓
c2(dilute) = 2,008 x 10-3 mol.dm-3
n(acid reacting) = cV = 2,008 x 10-3 x 25/1000✓ = 5,02 x 10-5 mol
n(base reacting) = 2 x 5,02 x 10-5 ✓= 1,004 x 10-4 mol
c(base) = n/V = 1,004 x 10-4/14,2 x 10-3 ✓ = 7,07 x 10-3 mol.dm-3✓
(6)
OPTION 2
[H3O+] = 10-1,3 mol.dm-3
[Acid] = ½ x10-1,3 ✓= 0,0251 mol.dm-3
c1V1= c2V2
(0,0251)(8) = c2 x 100✓
c2 = 2,008 x 10-3 mol.dm-3
caVa = na
cbVb nb
2,008 x 10-3 ( 25) ✓ = 1 ✓
cb x 14,2 ✓ 2
cb = 7,07 x 10-3 mol.dm-3 ✓
Range ( 7,04 x 10-3 to/tot 7,07 x 10-3 mol.dm-3)
7.3.2 B✓
Titration of a strong base and a strong acid ✓ (solution at end point neutral.)(2)
[22]
TOTAL: 150

