TECHNICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - 2018 JUNE EXAM PAST PAPERS AND MEMOS
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE
JUNE 2018
INSTRUCTIONS AND INFORMATION
Read the following instructions carefully before answering the questions.
- Write your FULL NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- Answer ALL the questions.
- Non-programmable calculators may be used.
- Appropriate mathematical instruments may be used.
- Number the answers correctly according to the numbering system used in this question paper.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Data sheet and periodic table are attached for your use.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write the letter (A–D) next to the question number(1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 The bending of light as it passes from one medium to another of different optical density, is called …
- reflection.
- refraction.
- dispersion.
- refractive index.
(2)
1.2 The electromagnetic radiation with the HIGHEST value of c/λ, where λ is the wavelength and c is the speed of electromagnetic waves in a vacuum, is …
- visible light.
- microwaves.
- gamma rays.
- ultraviolet rays.
(2)
1.3 Which ONE of the following is NOT a property of electromagnetic waves? Electromagnetic waves …
- are transverse.
- are longitudinal.
- can undergo refraction.
- travel at 3 x 108 m.s-1 in vacuum.
(2)
1.4 The diagram below shows light travelling in a semi-circular prism.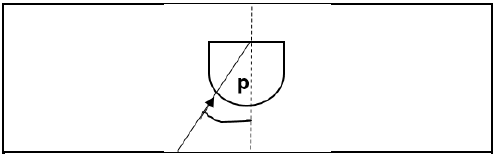
In which diagram is the angle p equal to the critical angle?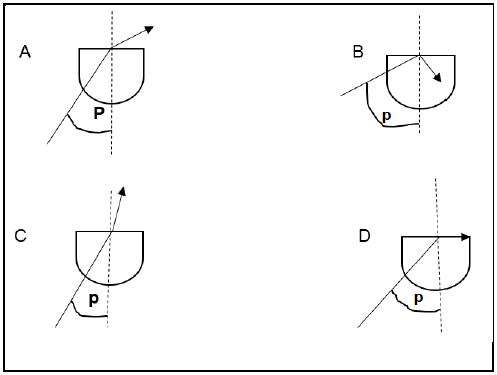
(2)
1.5 Which ONE of the following is a property of an image formed by a concave lens? The image formed by a concave lens is always …
- real and upright.
- real and inverted.
- virtual and inverted.
- virtual and upright.
(2)
1.6 Which ONE of the following is the general formula for alkanes?
- CnH4n
- CnH2n
- CnH2n+2
- CnH2n-2
(2)
1.7 Consider the compound.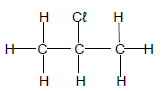
Which ONE of the following is the correct IUPAC name of this compound?
- Chloropropane
- 1-chloropropane
- 2-chloropropane
- 3-chloropropane
(2)
1.8 Consider the compound given below.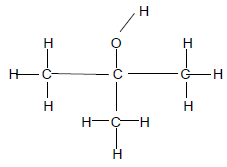
Which ONE of the following is TRUE about this compound? The compound is a …
- tertiary alcohol.
- primary alcohol.
- secondary alcohol.
- secondary haloalkane.
(2)
1.9 Which ONE of the following substances has the HIGHEST melting point?
- C4H8
- C4H10O
- C4H8O
- C4H12
(2)
1.10 A haloalkane reacts with water as shown in the balanced equation below.
CH3CH2CH2Br + H2O → CH3CH2CH2Br + HBr
Which ONE of the following CORRECTLY describes the type of reaction taking place?
- Addition
- Hydration
- Hydrolysis
- Polymerisation
(2)
[20]
QUESTION 2
The letters A to F in the table below represent six organic compounds
| A | O ║ CH3CHCCH3 | B | 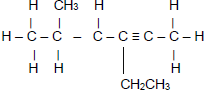 |
| C | 2,4- dimethylpentane | D | Butan-1-ol |
| E | 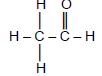 | F | C2H4O2 |
2.1 Write down only the letter(s) that represents EACH of the following:
2.1.1 An alkyne (1)
2.1.2 A ketone (1)
2.1.3 An aldehyde (1)
2.1.4 Hydrocarbons (2)
2.1.5 Alcohol (1)
2.2 To which homologous series do the following compounds belong?
2.2.1 Compound C (1)
2.2.2 Compound F (1)
2.3 Write down the IUPAC name of:
2.3.1 Compound A (2)
2.3.2 Compound B (3)
2.4 Write down the:
2.4.1 STRUCTURAL FORMULA of compound C (2)
2.4.2 Condensed STRUCTURAL formula of compound D (1)
2.5 Compound F is a structural isomer of ethanoic acid.
2.5.1 Define the term structural isomer. (2)
Compound F is prepared by reacting a carboxylic acid with an alcohol in the presence of concentrated sulphuric acid.
Write down the:
2.5.2 Name of the type of reaction (1)
2.5.3 Function of sulphuric acid in this reaction (1)
2.5.4 IUPAC name of the carboxylic acid (2)
2.5.5 STRUCTURAL formula and IUPAC name of the ester produced (4)
[26]
QUESTION 3
3.1 Learners carry out an investigation into the viscosities of three compounds A, B and C of equal volume at room temperature.
| A. Hexane B. Heptane C. Octane |
3.1.1 Define the term viscosity. (2)
3.1.2 Give a reason why the compounds A, B and C are said to be saturated. (2)
For the investigation write down the:
3.1.3 Independent variable (1)
3.1.4 Learners’ hypothesis (2)
The learners find out that the viscosity of compound C is the highest.
3.1.5 Explain fully why the viscosity of compound C is the highest. (3)
The three compounds A, B and C are components of petrol. Apart from undergoing combustion in a vehicle’s engine, the compounds also serve as lubricants.
3.1.6 Which ONE of the compounds (A, B or C) will be the best lubricant?
Give a reason in terms of the viscosity of the compound.(2)
3.1.7 Write down a balanced equation for the complete combustion of compound A using MOLECULAR formulae. (3)
3.2 Learners carry out an investigation into one of the factors that affects boiling point. The following table shows the boiling points obtained for alkanes and alcohols.
| ALKANE | bp (ºC) | ALCOHOL | bp (ºC) |
| Propane | -42 | Propan-1-ol | 97 |
| Butane | -0.5 | Butan-1-ol | 117 |
| Pentane | 36 | Pentan-1-ol | 138 |
| Hexane | 69 | Hexan-1-ol | 156 |
3.2.1 Name the instrument the learners used to measure the boiling points. (1)
3.2.2 Write down the relationship between chain length and the boiling point of alkanes. (2)
3.2.3 Use information from the table to predict the values between which the boiling point of 2-methyl propane lies. (1)
3.2.4 Which structural arrangement of the alcohol molecules makes the comparison of their boiling points a fair test? (2)
3.3 Explain fully in terms of intermolecular forces why the boiling points of the alcohols are higher than those of the corresponding alkanes. (3)
[24]
QUESTION 4
4.1 The flow diagram below shows different organic reactions using CH2=CH2 as the starting reagent. P, Q and R represent different organic compounds.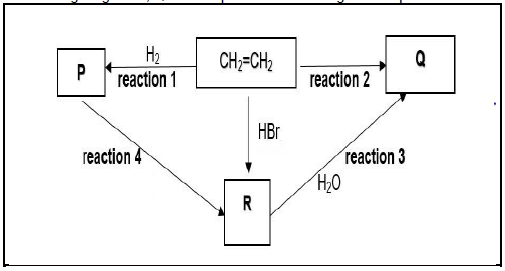
Write down the TYPE of:
4.1.1 Reaction represented by reaction 1 (1)
4.1.2 Addition reaction represented by reaction 2 (1)
4.1.3 Reaction represented by reaction 4 (1)
Write down the:
4.1.4 MOLECULAR formula of P (1)
4.1.5 NAME of the catalyst used in reaction 1 (1)
4.1.6 STRUCTURAL FORMULA of R (2)
4.1.7 Balanced equation for reaction 2 (4)
4.1.8 Name or formula of a base that can be used in place of water in reaction 3 (1)
4.1.9 Name or formula of the inorganic reactant used in reaction 4 (1)
4.1.10 ONE reaction condition for reaction 4 (1)
4.2 A wide range of synthetic polymers are produced by polymerisation.
Polymer P is an example of such a polymer.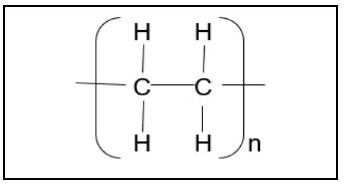
4.2.1 Define the term polymer. (2)
4.2.2 Write down ONE use of polymer P. (1)
Write down the:
4.2.3 STRUCTURAL FORMULA and IUPAC name of the monomer that is used to prepare this polymer (3)
4.2.4 Type of polymerisation reaction (ADDITION or CONDENSATION) that is used to prepare this polymer (1)
[21]
QUESTION 5
5.1 A ray of light travels from air to glass at an angle of incidence of 35o as shown in the diagram below. A and B represent the media through which the light is travelling.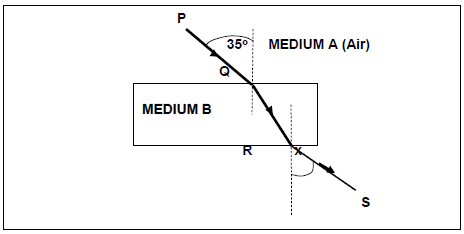
Write down the NAME of each of the following angles shown in the diagram:
5.1.1 The angle marked as 35o (1)
5.1.2 The angle marked as x (1)
5.2 Write down the value of angle x. (1)
5.3 Name the following:
5.3.1 Ray PQ (1)
5.3.2 Ray QR (1)
5.3.3 Ray RS (1)
5.4 Which ONE of the TWO media (A or B) is optically denser?
Explain your answer. (3)
[9]
QUESTION 6
6.1 When white light is shone onto a triangular glass prism, it undergoes dispersion as shown in the diagram below. (NOTE: Not all colours that make up the visible spectrum of light are shown in the diagram.)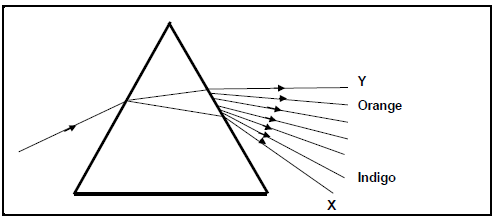
6.1.1 Define the term dispersion. (2)
6.1.2 Name the colours of visible light marked as:
- X (1)
- Y (1)
When light travels from air to glass, its speed decreases.
6.1.3 How does the wavelength of light change when light travels from air to glass?
Choose from INCREASES, DECREASES or REMAINS THE SAME
Explain your answer. (3)
6.2 Visible light forms a part of the electromagnetic spectrum. The diagram below shows some components of the electromagnetic spectrum and the corresponding wavelength range for each electromagnetic wave.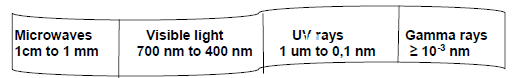
6.2.1 How are electromagnetic waves produced? (2)
6.2.2 What colour of visible light has a wavelength of 700 nm? (1)
6.2.3 Which ONE of the electromagnetic waves shown in the diagram …
- Has the HIGHEST energy in its photons? (1)
- Is used to sterilise food and appliances? (1)
- Can damage the skin of a person exposed to sunlight without protection? (1)
6.2.4 The electromagnetic spectrum is made up of seven electromagnetic waves.
In the diagram above, THREE of the electromagnetic waves in the electromagnetic spectrum are not shown.
- Write down the names of the THREE electromagnetic waves which are not shown in the diagram. (3)
- Arrange the THREE electromagnetic waves mentioned in QUESTION 6.2.4 (a) in order of increasing frequency.
(Start with the one with the lowest and finish with the highest frequency.) (2)
6.2.5 Electromagnetic waves are made up of photons.
Define the term photon. (2)
6.2.6 Calculate the:
- Maximum frequency of a photon of violet light (3)
- Minimum energy of a photon of gamma rays (4)
[27]
QUESTION 7
7.1 Define reflection. (2)
7.2 State the law of reflection. (2)
7.3 A learner carries out an experiment to determine the position of an image of an object formed by a plane mirror.
The diagram below shows the learner’s incomplete ray diagram.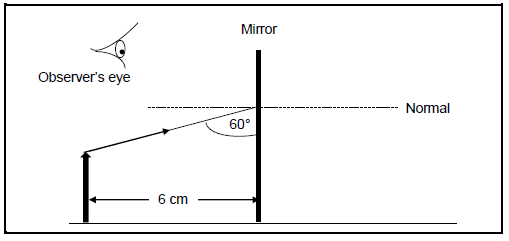
7.3.1 How does the size of the image compare to that of the object?
Choose from SMALLER THAN, HIGHER THAN or SAME SIZE. (1)
7.3.2 Write down the distance of the image from the object. (1)
7.3.3 Is the image formed by the plane mirror VIRTUAL or REAL? (1)
7.4 The diagram below shows a ray of light in a triangular glass prism.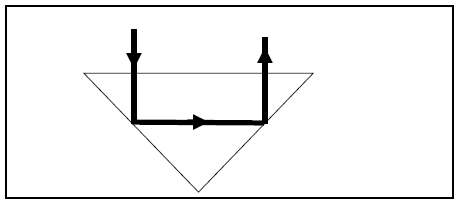
7.4.1 Name the phenomenon that light undergoes, as shown in the diagram. (1)
For the phenomenon named in QUESTION 7.4.1 above, write down:
7.4.2 The TWO conditions necessary for the phenomenon to happen (2)
7.4.3 A medical instrument used to examine internal parts of the body (1)
[11]
QUESTION 8
8.1 The diagram below shows the path of a light through a lens.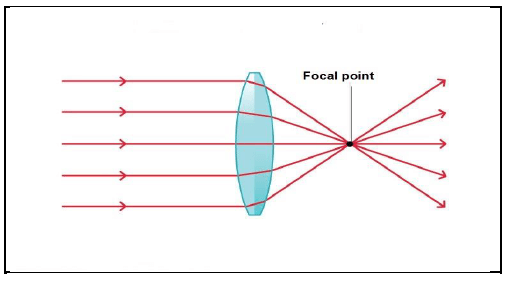
Is the lens CONCAVE or CONVEX?
Give a reason for your answer. (2)
8.2 A convex lens can be used as a magnifying glass.
8.2.1 Draw a ray diagram to show how a convex lens in a magnifying glass enlarges the image of an object. (6)
8.2.2 Give a reason why a concave lens cannot be used as a magnifying glass. (2)
8.3 Convex lenses find applications in photocopiers, cameras and projectors. Depending on the position of the object in front of the lens, images of different sizes are formed.
How will the size of an image compare to the size of the object when the object is placed in front of a convex lens:
8.3.1 Beyond 2F? (1)
8.3.2 Between F and 2F? (1)
[12]
TOTAL: 150
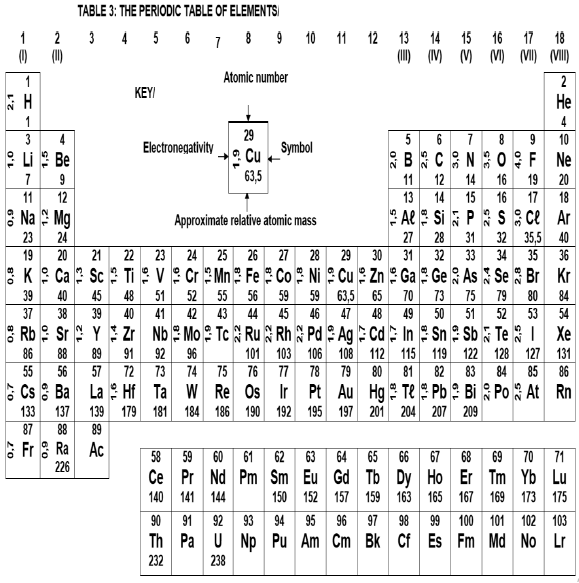
DATA FOR TECHNICAL SCIENCES GRADE 12
PAPER 2
TABLE 1
| PHYSICAL CONSTANTS | ||
| CONSTANT | SYMBOL | VALUE |
| Planck's constant | h | 6,63 x 10-34 J.s |
| Speed of light | c | 3 x 108 m.s-1 |
TABLE 2
| WAVES, SOUND AND LIGHT | |
| Speed | c = f λ |
| Energy | E = hf or E= hcλ |