Final Examination Revision Papers - Physical Sciences Grade 10 Study Guide
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPhysics Examination (Paper 1)
Data for Physical Sciences Grade 10 Physics (Paper 1)
Physical constants
Name | Symbol | Value |
Acceleration due to gravity | g | 9,8 m.s–2 |
Speed of light in a vacuum | c | 3,0 × 108 m.s–1 |
Charge on electron | e– | –1,6 × 10–19 C |
Planck’s constant | h | 6,63 × 10–34 J.s |
Formulae
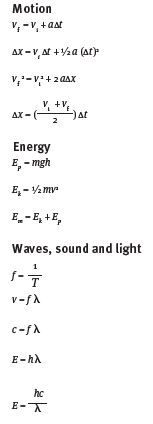

SECTION A
QUESTION 1: ONE-WORD ANSWERS
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
1.1 The rate of change of position.
1.2 The quantity represented by the area under a velocity vs. time graph.
1.3 The type of wave in which the vibrations of the particles is at right angles to the direction in which the wave is travelling.
1.4 The way in which a voltmeter is connected in a circuit.
1.5 A quantum of visible light. [5]
QUESTION 2: MATCHING PAIRS
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 the principle of adding together the amplitudes of waves that meet, so as to find the resultant amplitude 2.2 the highest frequency of electromagnetic radiation 2.3 magnetic field lines 2.4 gradient of a velocity vs. time graph 2.5 kinetic energy of an object falling in the absence of air friction |
|
[5]
QUESTION 3: TRUE/FALSE
3.1 The electrical resistance of a conductor depends only on the length and the thickness of the conductor.
3.2 For a car travelling from rest and at constant acceleration, its displacement is directly proportional to its time of travel.
3.3 When resistors are connected in series they act as dividers of potential difference.
3.4 The area under a velocity vs. time graph represents the acceleration of the object.
3.5 When an object falls vertically in the absence of air friction, its mechanical energy remains constant. 5 × 2 = [10]
QUESTION 4: MULTIPLE CHOICE
4.1 In which of the following situations will the distance covered and the magnitude of the displacement of a car be the same?
- A girl runs in a straight line across a level field.
- A car travels half the circumference of a circular race track.
- A car travels from Cape Town to Johannesburg.
- A car travels the full circumference of a circular race track. (3)
4.2 Consider the accompanying velocity–time graph of a child riding her bicycle.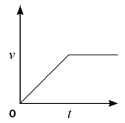
The corresponding graph, representing the acceleration of the bicycle vs. the time is: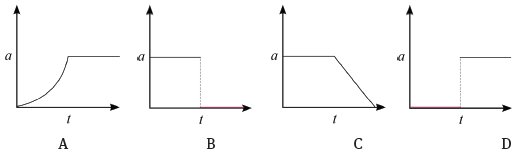 (3)
(3)
4.3 A bungi jumper of mass m falls freely from a bridge of height h. He reaches a velocity v after falling a distance x. If air resistance is negligible, his total mechanical energy at this point is:
- mg(h–x).
- ½ mv2.
- mgh + ½ mv2.
- mgh. (3)
4.4 When resistor R1 and R2 are connected in parallel, the total resistance of the combination is always:
- very much larger than either R1 or R2.
- equal to R1 + R2.
- equal to R1 × R2.
- smaller than the smaller of R1 and R2. (3)
4.5 A source of frequency 500 Hz emits waves of wavelength 0,2 m. How long does it take the waves to travel 300 m?
- 3 s
- 12 s
- 60 s
- 100 s (3) [15]
SECTION B
QUESTION 1
1.1 Distinguish between a vector quantity and a scalar quantity. (2)
1.2 What is meant by the ‘resultant’ of two forces? (2)
1.3 A box, on the floor, is being pulled by two boys by means of ropes tied to the box. The forces that they apply are 70 N and 50 N. What is the minimum resultant force that the ropes can exert on the box, and what must be the angle between the ropes in order for them to exert this minimum force? (2) [6]
QUESTION 2
The two boys apply the same forces as in question 1 on the box by means of ropes. The 70 N force is exerted in a direction 90°, while the 50 N force is exerted in a direction 180°.
2.1 By means of an accurate scale drawing, using the tail-to-tail method, determine the magnitude and direction of the resultant force being exerted on the box. (Use a scale of 10 mm = 10 N.) (7)
2.2 Verify your answer by using mathematical and trigonometrical calculations to determine the resultant force. (6) [13]
QUESTION 3
The brakes of a car are being tested on a straight, level tarred road. At the far end of the road is a stretch of soft sand to stop the car, should it not stop in time. The length of the road up to the sand is 192 m. The car accelerates uniformly at 9,8 m.s–2 from rest at the start of the road. After travelling 122,5 m, the brakes are applied and the car slows down uniformly, coming to rest 10 m from the sand.
3.1 Define acceleration. (2)
3.2 Calculate the time taken for the car to travel the first 122,5 m. (4)
3.3 What is the velocity of the car at the 122,5 mark? (4)
3.4 Calculate the acceleration of the car during the time that the brakes are applied. (5)
3.5 The speedometer of a car registers ‘instantaneous speed’. How is instantaneous speed measured? (3) [18]
QUESTION 4
The following velocity–time graph represents the motion of a trolley on a track. In carrying out the re- quired calculations do not use equations of motion.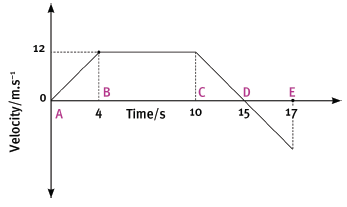
4.1 Calculate from the graph the acceleration of the trolley for the time interval CD. (4)
4.2 Describe (in words) the motion of the trolley for the interval ABC. (3)
4.3 What happened to the trolley during time interval DE? (3)
4.4 Calculate the displacement of the trolley during the interval ABC. (5)
4.5 Draw a sketch of the corresponding acceleration–time graph. Show the time values (0 s to 17 s) on the time axis, but do not show any acceleration values on the Y-axis. (6) [21]
QUESTION 5
Moneeb and his little sister Moneeba are playing with a toy car of mass 3 kg on the slide in the school playground. The toy car is moving at a speed of 2,63 m.s–1 when it passes point P. Point P is 0,75 m above point R, the lowest position of the slide. Ignore all types of friction. Express answers correct to two decimal places.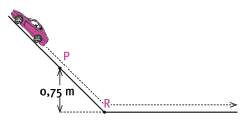
5.1 State what is meant by the ‘mechanical energy’ of an object. (2)
5.2 Under what condition is the mechanical energy of a falling object conserved? (1)
5.3 Calculate the total mechanical energy of the car at point P. (6)
5.4 What is the kinetic energy of the car at point R? (2)
5.5 Calculate the velocity of the car at point R. (4) [15]
QUESTION 6
Two pulses, P and Q in a string, approach each other at the same speed. Pulse P has an amplitude of +4,0 cm when it is at position X. Pulse Q has an amplitude of –6,0 cm when it is at position Z. Points X and Z are the same distance from point Y. The pulses both have a length of 8,0 cm. Pulses P and Q meet at position Y. Assume that no energy is lost.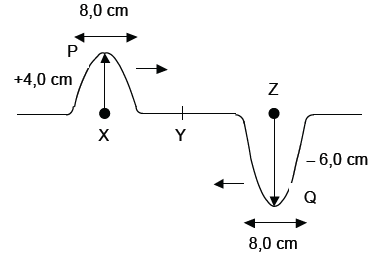
6.1 Write down the definition of a pulse. (2)
6.2 Make a labelled sketch to show what happens when the pulses P and Q meet at position Y. Also indicate the pulse length. (4)
6.3 A transverse wave is formed by a succession of pulses each with amplitude 4 cm and pulse length 8 cm (similar to P above). If the period of this transverse wave is 0,4 s, calculate the
velocity of the wave in m.s–1. (6) [12]
QUESTION 7
Water waves are travelling towards the concrete wall of a harbour, as shown in the sketch below. Six wavelengths strike the wall in 24 seconds. The distance between successive crests is 10 m. The height of the waves from trough to crest is 2,5 m.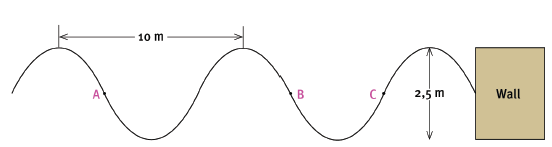
7.1 Are points A and C ‘in phase’? Explain your answer. (3)
7.2 The waves are moving towards the wall, from left to right. In what direction is point B on the wave moving? (2)
7.3 What is the amplitude of the wave? (2)
7.4 Calculate the period of the wave. (3)
7.5 Calculate the frequency of the wave. (3) [13]
QUESTION 8
Pianos, guitars and other stringed musical instruments have to be ‘tuned’. This means that the tension in the strings must be adjusted so that they produce the correct note when struck or plucked. This is done by matching the sound produced by the instrument to that produced by a ‘tuning fork’. A particular tuning fork is marked 440 Hz.
8.1 What is meant by the marking 440 Hz? (2)
8.2 What type of wave is a sound wave? (2)
8.3 The speed of sound at sunrise at a particular place is 330 m.s–1. Would you expect the speed to be faster or slower at midday when it is much hotter? (2)
8.4 When sound travels from air into water, what happens to the speed of the sound? Give a reason for you answer. (4) [10]
QUESTION 9
When we see a rainbow, we are seeing a small part of a very wide range of wavelengths called an ‘electromagnetic spectrum’.
9.1 What are the names of the portions of the electromagnetic spectrum on either side of the visible spectrum? (2)
9.2 Explain what contribution Max Planck made to our understanding of electromagnetic waves? (4)
9.3 Explain why visible light is considered to have a ‘dual nature’. (6)
9.4 The energy of a quantum of electromagnetic radiation is 3,55 × 10–19 J. Calculate the wavelength of the wave. (5)
9.5 Express the answer to 9.4 in nanometres. (2) [19]
QUESTION 10
A plastic ruler is rubbed vigorously with a cloth. As a result, the ruler has become negatively charged. The ruler is then held close to a small bead covered with thin metal foil, as shown in the sketch.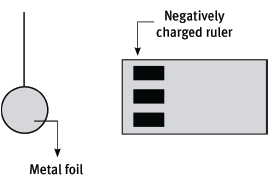
10.1 Why has the ruler become negatively charged? (2)
10.2 The charge on the ruler is –1,36 × 10–12 C. Calculate the number of excess electrons on the ruler. (4)
10.3 The bead is attracted by the ruler. Explain why it is attracted. (4)
10.4 What would you expect to see after the bead has touched the ruler? (2)
10.5 Explain why this should occur. (3) [15]
QUESTION 11
Consider the circuit diagram given below. You are given that V1 reads 12 V, A1 reads 3 A and V2 reads 9 V. Resistors R1 and R2 are identical.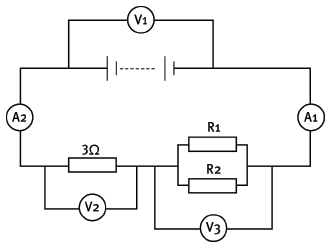
11.1 Define potential difference. (2)
11.2 What is the reading on V2? (2)
11.3 What is the reading on A2? (2)
11.4 You are given that the single-series resistor has a resistance of 3 Ω. Explain why the effective resistance of the parallel pair of resistors is 1 Ω. (4)
11.5 What is the resistance of R1? (2)
11.6 Calculate the charge that passes through the 3 Ω resistor in 2 minutes. (4) [16]
QUESTION 12
You are given this labelled circuit diagram.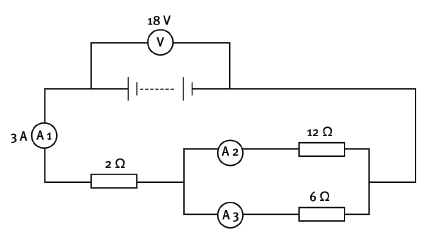
12.1 Calculate the equivalent resistance of the whole circuit. (5)
12.2 If the reading on A3 is 2 A, what is the reading on A2? (2) [7]
Answers
SECTION A
QUESTION 1: ONE-WORD ANSWERS
1.1 velocity
1.2 displacement
1.3 transverse
1.4 series
1.5 photon
QUESTION 2: MATCHING PAIRS
2.1 B
2.2 D
2.3 C
2.4 G
2.5 E
QUESTION 3: TRUE/FALSE
3.1 False. Electrical resistance depends on the type of material, length, thickness and temperature.
3.2 False. For a car travelling from rest and at constant acceleration, its displacement is directly proportional to the square of its time of travel.
3.3 True.
3.4 False.The area under a velocity vs. time graph represents the displacement of the object.
3.5 True.
QUESTION 4: MULTIPLE CHOICE
4.1 A
4.2 B
4.3 D
4.4 D
4.5 A
SECTION B
QUESTION 1
1.1 A vector quantity has both magnitude and direction, while a scalar quantity has magnitude only.
1.2 The resultant of a number of vectors is the single vector that has the same effect as all the vectors acting together.
1.3 20 N 180°
QUESTION 2
2.1 Scale: 10 mm = 10 N
(Correct length of 70 N)
(Correct length of 50 N)
Resultant force = 86 N (+ –2 N) 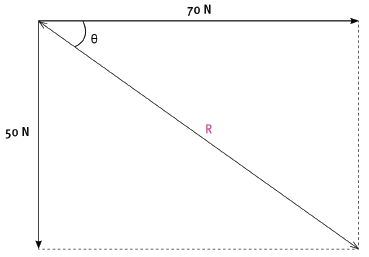
θ = 36° (+ –2°)
2.2 Using Pythagoras:
- R2 = 802 + 602
R = 86,0 N
Sin θ = opp = 50
hyp 86
θ = 35,6°
Direction = 125,6°
QUESTION 3
3.1 The rate of change of velocity.
3.2
- Δx = viΔt + ½ a(Δt)2
122,5 = 0 + ½ (9,8) (Δt)2
(Δt)2 = 122,5 = 25
4,9
Δt = 5 s
3.3
- vf = vi + aΔt OR vf2 = vi2 + 2 aΔ x
= 0 + (9,8) (5) = 0 + 2(9,8) (122,5)
= 49 m.s–1 = 2401
vf = 49 m.s–1
3.4 Distance travelled while brakes are applied
- = 192 – 122,5 – 10 = 59,5 m
vf2 = vi2 + 2 aΔx
0 = 492 + 2(a)(59,5)
a = −(492)
119
a = –20,2 m.s–2
3.5 By measuring the distance travelled during a very short time interval and applying the formula v = D/Δt
QUESTION 4
4.1 Acceleration =
- gradient = ∆y = 12
∆x 5
= –2,4 m.s–2
4.2 Accelerated uniformly from rest for 4 seconds then continued at constant velocity of 12 m.s–1 for a further 6 s.
4.3 Trolley reversed from rest with a uniform acceleration for 2 seconds.
4.4 Displacement = area under graph
- = ½ bh + lb
= ½ (4) (12) + (6) (12)
= 96 m
4.5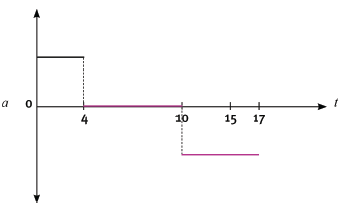
Graph 0 s – 4 s correct
Graph 4 s – 10 s correct
Graph 10 s – 17 s correct
QUESTION 5
5.1 The sum of the kinetic energy and potential energy.
5.2 zero friction
5.3 Mechanical energy
- = Ep + Ek
= mgh + ½ mv2
= 3 (9,8) (0,75) + ½ (3) (2,63)2
= 32,43 J
5.4 At R the Ep = 0. So Ek = EM = 32,43 J
5.5
- Ek = ½ mv2
v2 = 2 Ek = 2 (32,43) = 21,62
m 3
v = 4,65 m.s–1
QUESTION 6
6.1 A single disturbance in a medium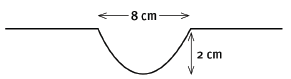
Pulse drawn correctly
Correct amplitude
Correct pulse length
6.3
- f = 1 = 1 = 2,5 Hz
T 0,4
Pulse length = 8 cm. Wavelength = 16 cm = 0,16 m
v = f λ = (2,5) (0,16)
= 0,4 m.s–1
QUESTION 7
7.1 No - They are not ‘in step’. When A is moving up, C is moving down.
7.2 Vertically upwards
7.3 1,25 m
7.4 If 6 wavelengths strike the wall in 24 s, one wavelength will strike every 4 s.
7.5
- f = 1 = 1
T 4
= 0,25 Hz
QUESTION 8
8.1 440 vibrations per second
8.2 longitudinal waves
8.3 faster
8.4 speed increases Water molecules are much closer together than air molecules, so the energy is transferred more quickly from molecule to molecule.
QUESTION 9
9.1
- infrared
ultraviolet
9.2
- Energy is not radiated continuously but in packages named ‘quanta’.
- The energy content of a quantum is directly proportional to the frequency of the wave.
9.2 A quantum of energy is similar to a particle, but within the quantum there is an electromagnetic wave. At the radio wave end of the spectrum, the wavelength is so long that the waves are almost continuous – the ‘wave nature’. At the gamma ray end the wavelength is so short that the wave nature is not noticeable – the quantum behaves as a particle. Visible light is in the middle of the whole spectrum, so both wave nature and particle nature are evident.
9.4
- E = hc λ = hc = (6,63 × 10–34) (3 × 108)
λ λ 3,55 × 10–19
= 5,6 × 10–7 m
9.5 560 nm
QUESTION 10
10.1 Electrons have been rubbed off the cloth, onto the ruler.
10.2
- Q = nqe n = Q = 1,36 × 10 – 12
qe 1,6 × 10 – 19
= 8,5 × 106 electrons
10.3 Loosely bound electrons in the metal foil are repelled to the far side of the bead, making that side negatively charged, and leaving the side closest to the ruler positively charged (that is, the bead is polarised). The force of attraction between the negative ruler and the positive side of the bead is stronger than the repulsion between the ruler and the negative side because the positive side is closer. So the bead is attracted.
10.4 The bead is repelled.
10.5 Some electrons are transferred from the ruler onto the bead, making the whole bead negatively charged. The ruler is still charged negatively. The two negatively charged objects repel.
QUESTION 11
11.1 energy transferred per coulomb of charge
11.2 3 V
11.3 3 A
11.4 Resistors in series divide the voltage of the battery in proportion to their resistances. The voltage across the parallel combination is one-third of the voltage across the 3 Ω resistor. Therefore, its resistance must also be one-third. Therefore 1 Ω.
11.5 2 Ω
11.6
- Q = IΔt
= (3) (120)
= 360 C
QUESTION 12
12.1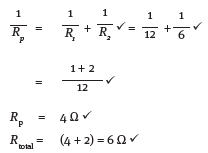
12.2 1 A
Paper 2
Data for Physical Sciences Grade 10 Chemistry (Paper 2)
Physical constants
Name | Symbol | Value |
Avogadro’s number | NA | 6,02 × 1023 |
Molar gas volume | 22,4 dm3.mol–1 | |
Standard temperature | 273 K (0 °C) | |
Standard pressure | 101,3 kPa |
Formulae
n = m
M
c = n
V
c = m
MV
SECTION A
QUESTION 1: ONE-WORD ANSWERS
Provide one word or term for each of the following descriptions. Write only the word or term next to the question number.
1.1 When a liquid changes from a liquid into a gas. (1)
1.2 All water on and around the Earth. (1)
1.3 A chemical change that causes the surroundings to get cooler. (1)
1.4 The energy required to remove one electron from an atom of an element in the gaseous phase. (1)
1.5 The attraction that exists when two atoms share the same pair of electrons. (1) 5 × 1 = [5]
QUESTION 2: MATCHING PAIRS
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 property of a metal | A number of protons |
2.2 mass number | B number of electrons in the outer shell |
2.3 valency | C kilogram |
2.4 CaCO3 + heat → CaO + CO2 | D number of protons and neutrons |
2.5 SI unit for quantity of matter | E decomposition reaction |
F ductile | |
G mole | |
H brittle | |
I number of chemical bonds that an atom can form | |
J synthesis reaction |
5 × 1 = [5]
QUESTION 3: TRUE/FALSE
Indicate whether each of the following statements is true or false.
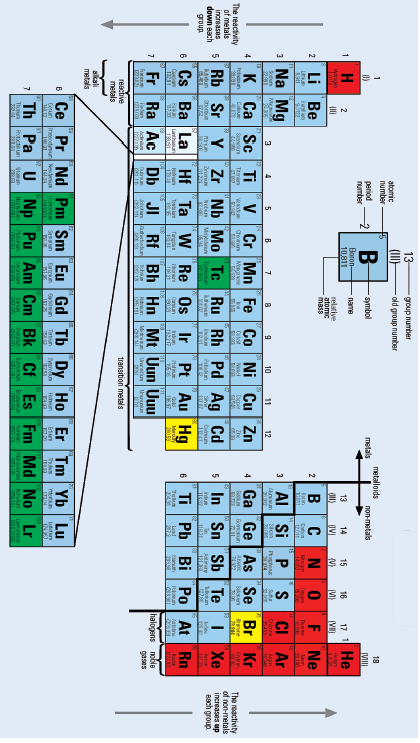
3.1 Elements in the same horizontal row in the periodic table have the same number of electrons in their outer energy levels.
3.2 Covalently bonded substances do not usually conduct electricity.
3.3 Oxidation is the process of water molecules surrounding ions when an ionic solid dissolves in water.
3.4 The chloride ion and the potassium ion have the same number of electrons.
3.5 Salt can become a conductor of electricity either by dissolving it in water or by melting it. 5 × 2 = [10]
QUESTION 4: MULTIPLE CHOICE
Choose the correct answer. Only write the letter of the answer that you select.
4.1 Atoms form bonds when valence electrons interact. When electrons are transferred from one atom to another the bond is:
- ionic.
- covalent.
- metallic.
- electronic. (3)
4.2 When sodium chloride dissolves in water, the . . . of the water molecule is attracted by the chlo- ride ion.
- hydrogen end, which is the positive pole
- hydrogen end, which is the negative pole
- oxygen end, which is the positive pole
- oxygen end, which is the negative pole (3)
4.3 This shows a representation of the nuclei of two atoms: ![]() . These atoms:
. These atoms:
- have the same mass numbers.
- are isotopes of the same element.
- have the same number of neutrons.
- have the same number of electrons. (3)
4.4 X represents some imaginary element with a fixed valency. The other elements below exhibit their normal valencies. Which one of the following formulae is wrong?
- XCl3
- X2S3
- X2O3
- X(NO3)2 (3)
4.5 Which set of coefficients will balance the following equation?
Al + H2O → Al2O3 + H2
- 2 : 1 : 3 : 3
- 2 : 3 : 1 : 3
- 3 : 2 : 1 : 3
- 2 : 3 : 3 : 1 (3) 3 × 5 = [15]
SECTION B
QUESTION 1
The following table shows the first ionisation energies for the elements of periods 1 and 2.
Period | Element | First ionisation energy (kJ.mol–1) |
1 | H | 1 312 |
He | 2 372 | |
2 | Li | 520 |
Be | 899 | |
B | 801 | |
C | 1 085 | |
N | 1 402 | |
O | 1 314 | |
F | 1 681 | |
Ne | 2 081 |
1.1 What is the meaning of the term ‘first ionisation energy’? (2)
1.2 Identify the general pattern of first ionisation energies in a period. (2)
1.3 Which two elements on the table above exert the strongest forces of attraction on their electrons? What property concerning the electrons in their outer shells is responsible for this? (4)
1.4 ‘All group 1 elements readily form positive ions’. Is this statement correct? Explain your answer by referring to the table. (3) [11]
QUESTION 2
Ernest Rutherford carried out one of the most important experiments in the history of science. He bombarded a very thin sheet of gold with fast-moving positively charged particles. He was able to trace the paths of the particles by seeing where they hit a spherical glass screen. From the results of his experiment, he was able to propose a new model for the structure of an atom.
2.1 Briefly describe the results of his experiment. (4)
2.2 Briefly describe the model of the atom that he was able to propose, based on these results. (4) 2 × 4 = [8]
QUESTION 3
A neutral atom has 20 electrons around its nucleus.
3.1 To which element does this atom belong? (2)
3.2 How many valence electrons does it have? (2)
3.3 How many energy levels does it have? (2)
3.4 Will this atom gain or lose electrons when it forms an ionic bond? Explain. (3)
3.5 How many electrons will this atom gain or lose during ionic bonding? (2)
3.6 After this atom has gained/lost electrons, which noble gas will have the same electron structure as the ion that is formed? (2) [13]
QUESTION 4
The element chlorine exists as three isotopes, one of which is 3517Cl.
4.1 What are ‘isotopes’ of an element? (2)
4.2 How many protons does the above isotope have? (2)
4.3 How many neutrons does it have? (2)
4.4 The other isotope of chlorine has 20 neutrons. Write its structure using the same notation as above. (2)
4.5 The atomic mass of chlorine is 35,5. What does this tell us about the ratio in which the two isotopes of chlorine occur in nature? (2) [10]
QUESTION 5
Potassium metal will burn in oxygen to form potassium oxide.
5.1 State Pauli’s exclusion principle. (2)
5.2 Use the ‘arrows in boxes’ notation to show the electron configuration of an oxygen atom. Label the energy levels and orbitals. (4)
5.3 Use Lewis structures to show the ionic bonding between potassium and oxygen atoms. (4)
5.4 Write the balanced chemical equation for the reaction between potassium and oxygen. (3) [13]
QUESTION 6
Sodium chloride is soluble in water.
6.1 Draw the Lewis structure for a water molecule. (3)
6.2 What is meant by the ‘electronegativity’ of an element? (2)
6.3 Instead of using a pair of dots or a dot and a cross to represent a shared pair of electrons in a covalent bond, a single line may be used. This method is called a Couper structure. Draw the Couper structure for a water molecule, showing the approximate bond angle in the structure. (2)
6.4 Explain why the water molecule is a dipole. Use the Couper structure sketch to illustrate your answer. (4)
6.5 Explain briefly the process that occurs when sodium chloride dissolves in water. Include in your explanation a sketch showing the positions of the water molecules around the ions. (7)
6.6 Write a chemical equation, including the phases, to represent this dissolution process. (3) [21]
QUESTION 7
You are given the following solubility rules:
- All nitrates are soluble.
- Salts of sodium, potassium and ammonium are soluble.
- All chlorides are soluble, except silver chloride and lead chloride.
- All sulphates are soluble, except the sulphates of calcium, barium, lead and silver.
- All carbonates are insoluble, except the carbonates of sodium, potassium and ammonium.
- All hydroxides are insoluble, except the hydroxides of sodium, potassium and ammonium.
Certain chemical reactions are classified as ‘precipitation reactions’.
7.1 What is a precipitate in this context? (2)
7.2 Is lead chloride soluble in water? (1)
7.3 You mix together solutions of sodium nitrate and potassium sulphate. Will a precipitate form? (Answer simply Yes or No.) (2)
7.4 You mix together solutions of silver nitrate and potassium chloride. A precipitate is formed.
7.4.1 Write down the name of the precipitate. (2)
7.4.2 Write a chemical equation for this reaction, including the states/phases. (4)
7.4.3 This is an example of a ‘precipitation reaction’. What other classification name applies to this reaction? (2) [13]
QUESTION 8
Write chemical formulae for the following compounds:
8.1 aluminium sulphate (2)
8.2 calcium hydroxide (2)
8.3 ammonium carbonate (2)
8.4 silver phosphate (2)
8.5 beryllium sulphide (2) 5 × 2 = [10]
QUESTION 9
Write balanced chemical equations for the following reactions:
9.1 hydrogen sulphate + sodium carbonate → sodium sulphate + carbon dioxide + water (5)
9.2 potassium + hydrogen oxide (water) → potassium hydroxide + hydrogen (5) [10]
QUESTION 10
Balance the following equations:
10.1 P + Cl2 → PCl3 (2)
10.2 Mg2SiO4 + H2O → Mg(OH)2 + H4SiO4 (2) [4]
QUESTION 11
Copper (II) sulphate (CuSO4) is formed when copper (II) oxide reacts with sulphuric acid. Water is the other product in this reaction. (Express answers correct to two decimal places.)
11.1 Write a balanced chemical equation for the reaction between copper (II) oxide and sulphuric acid. (4)
11.2 Calculate the percentage copper in copper (II) sulphate. (5)
11.3 Calculate the mass of copper (II) sulphate that must be dissolved in water to make up 250 cm3 of solution of concentration 0,4 mol.dm–3 (5) [14]
QUESTION 12
The empirical formula of a compound is found to be HO
12.1 What is meant by the ‘empirical formula’ of a compound? (2)
12.2 The formula mass of this compound is 34. What is the molecular formula of this compound? (2)
12.3 A compound was analysed by a chemist, who found that the compound contained 31,8 g potas- sium, 29,0 g chlorine and 39,2 g oxygen. Calculate the empirical formula of the compound. (6) [10]
QUESTION 13
You are given the following balanced chemical equation. (Express answers correct to 2 decimal places.)
CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
13.1 Calculate the mass of CaCl2 that will be obtained if 17 g of CaCO3 is reacted completely with HCl. (5)
13.2 Calculate the volume of CO2, measured at STP, that will be obtained. (5)
13.3 Why is it necessary for the volume of the gas to be measured at a particular temperature and pressure? (2) [10]
QUESTION 14
For this question, you may refer to the solubility rules given in question 7. You have a solution of barium chloride in a reagent bottle. You are given three test tubes: A, B and C. They contain the following solutions:
- copper sulphate
- sodium nitrate
- potassium carbonate
You add some barium chloride solution to each test tube.
14.1 In which two test tubes will you see a precipitate? (2)
14.2 Name the precipitates formed. (4)
14.3 What will you see if a solution of nitric acid is added to each test tube containing precipitates? (3)
14.4 Write an equation for the reaction described in 14.3. (3) [12]
QUESTION 15
15.1 Ammonia is a gas that has a very strong smell that actually hurts your nose and throat a bit and makes your eyes water. In terms of the kinetic molecular theory, explain why diffusion takes place when ammonia gas is released in a classroom and eventually every member of the class can smell the gas. (3)
15.2 What is meant by ‘sublimation’? Give an example of a substance that sublimes. (3) [6]
Total marks: 200
Answers
SECTION A
QUESTION 1: ONE-WORD ANSWERS
1.1 evaporates (or boils)
1.2 hydrosphere
1.3 endothermic
1.4 ionisation energy
1.5 covalent bond
QUESTION 2: MATCHING PAIRS
2.1 F
2.2 D
2.3 I
2.4 E
2.5 G
QUESTION 3: TRUE/FALSE
3.1 False
3.2 True
3.3 False
3.4 True
3.5 True
QUESTION 4: MULTIPLE CHOICE
4.1 A
4.2 A
4.3 C
4.4 D
4.5 B
SECTION B
QUESTION 1
1.1 First ionisation energy is the amount of energy required to remove the outermost (or first) electron from an atom in the gaseous phase.
1.2 Increases
1.3 He and Ne . They both have full outer shells.
1.4 No. A lot of energy is required to remove an electron from H, so H usually forms covalent bonds.
QUESTION 2
2.1 While nearly all the charged particles went straight through the gold, some were deviated from their path and a very small number were even reflected straight back.
2.2 The fact that nearly all went straight through meant that most of the atom must be empty space. Deviation was caused by repulsion of positive charge, so the positive charge and most of the mass of the atom is concentrated in a very small space, the nucleus. The electrons surround the nucleus to make up the volume of the atom.
QUESTION 3
3.1 Ca
3.2 3
3.3 3
3.4 Lose electrons. It is a metal, which has a weak attraction for outer shell electrons.
3.5 2
3.6 Ar
QUESTION 4
4.1 Isotopes are atoms of the same element that have the same atomic number but different mass number (or the same number of protons, but a different number of neutrons).
4.2 17
4.3 18
4.4 3717 Cl
4.5 The ratio of Cl–35 to Cl–37 must be approximately 1:3.
QUESTION 5
5.1 The maximum number of electrons that an orbital can contain is 2.
5.2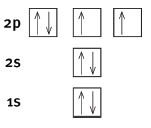
- Correct arrows and boxes
- Correct labelling
5.3![]()
- Correct reactants
- Correct product
5.4 2 K + O2 → K2O
QUESTION 6
6.1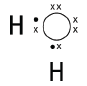
6.2 Electronegativity is the force of attraction that an atom has on a shared pair of electrons in a covalent bond.
6.3 Show bond angle > 90° 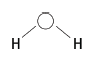
6.4 The electronegativity of O is greater than that of H. So, the shared pair of electrons is closer to the O than the H atoms, making the O end slightly negative and the H ends slightly positive. Couper structure 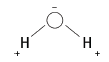
6.5 The O end of water molecules attract the H+ ions, while the H ends attract the Cl– ions. This causes the NaCl crystals to dissociate (or separate) into Na+ and Cl– ions. The water molecules now surround the ions, as in the sketch. This process is called hydration. Sketch 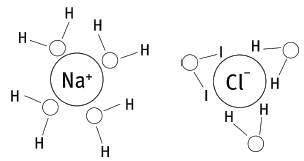
6.6 NaCl(s) → Na+(aq) + Cl–(aq)
QUESTION 7
7.1 A solid, insoluble substance
7.2 No
7.3 No
7.4.1 Silver chloride
7.4.2 AgNO3(aq) + KCl(aq) → AgCl(s) + KNO3(aq)
7.4.3 Ion exchange
QUESTION 8
8.1 Al2(SO4)3
8.2 Ca(OH)2
8.3 (NH4)2CO3
8.4 Ag3PO4
8.5 BeS
QUESTION 9
9.1 H2SO4 + Na2CO3 → Na2SO4 + CO2 + H2O
9.2 2 K + 2 H2O → 2 KOH + H2 (Correct balance )
QUESTION 10
10.1 2 P + 3 Cl2 → 2 PCl3
10.2 Mg2SiO4 + 4 H2O → 2 Mg(OH)2 + H4SiO4
QUESTION 11
11.1 CuO + H2SO4 → CuSO4 + H2O
11.2 Formula mass
- CuSO4 = 63,5 + 32 + 64 = 159,5
% Cu - 63,5 ×100
159,5
= 40,81%
11.3
- c = m m - MV - 159,5 x 0,25
MV c 0,4
= 99,70 g
QUESTION 12
12.1 The empirical formula is the simplest ratio of the atoms in the molecule or formula unit.
12.2 H2O2
12.3 In 100 g of the compound, we have 31,8 g K; 29,0 g Cl; 39,2 g O.
- Using n = M we have 31,8 mol K ; 29,0 mol Cl ; 39,2 mol O
M 39 35,5 16
= 0,82 mol K; 0,82 mol Cl; 2,45 mol O
Ratio = 1 : 1 : 3
Empirical formula = KClO3
QUESTION 13
13.1
- CaCO3 + 2 HCl → CaCl2 + CO2 + H2O
1 mol CaCO3 forms 1 mol Ca Cl2
100 g Ca CO3 forms 111 g Ca Cl2
17 g CaCO3 forms x g CaCl2
x = 17 × 111 = 18,87 g
100
13.2
- 1 mol CaCO3 forms 22,4 dm3 CO2 at STP
100 g CaCO3 forms 22,4 dm3 CO2 at STP
17 g CaCO3 forms x dm3 CO2 at STP
x = 17 × 22,4 = 3,81 dm3
100
13.3 Volume increases as temperature increases and decreases as pressure decreases.
QUESTION 14
14.1 A and C
14.2 Barium sulphate and barium carbonate
14.3
- Barium sulphate precipitate is not affected.
Barium carbonate precipitate disappears and bubbles of gas are seen.
14.4 CaCO3 + 2 HNO3 → Ca(NO3)2 + CO2 + H2O [12]
QUESTION 15
15.1 Gas particles are moving at high speed in all directions, colliding with one another and any- thing in their path. There are very big spaces between the particles of air, so the ammonia molecules move through these spaces until they are evenly spread in the classroom.
15.2 Sublimation is the change from solid to gas without going through the liquid phase. Examples include solid CO2 (dry ice), naphthalene, and so forth.