Chemical Change - Physical Science Grade 10 Study Guide
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupOverview
Summary
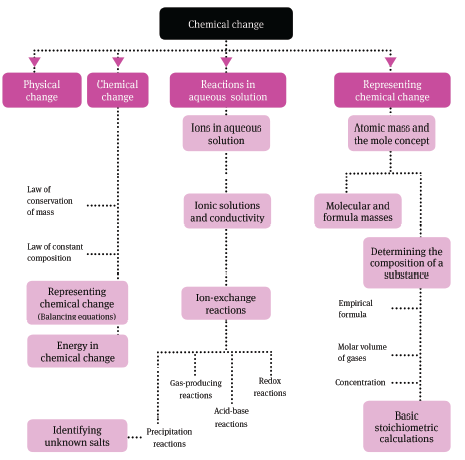
1 Physical and chemical change
1.1 Characteristics of physical and chemical change
- A physical change is usually easy to reverse. No new chemical substance is formed; usually only a small amount of energy is involved.
- During a physical change, the mass, the number of atoms and the number of molecules remain constant. Only intermolecular forces are broken.
- An example of physical change is ice melting.
- A chemical change is usually hard to reverse. New substances are formed that have different properties from the original substances. Usually, a large amount of energy is involved in the change.
- During a chemical change, mass and the number of atoms remain constant but the number and type of molecules will change. The atoms break apart and rearrange to form new compounds.
- An example of chemical change is iron sulphide being formed from the heating together of iron and sulphur.
- When only a physical change is involved, separation methods such as filtration, distillation and paper chromatography can be used to separate a mixture. Once a chemical change has occurred, these methods cannot be used.
1.1.1 Law of conservation of matter
- The total mass of any isolated system is constant and is independent of any chemical and physical changes taking place within the system.
1.1.2 Law of constant composition
- A particular chemical compound always contains the same elements combined in the same fixed proportions by mass.
- You can work out the ratio in which the elements combine by looking at their atomic masses.
1.2 Representing chemical change
- Chemical change can be represented or shown by balanced chemical equations. A balanced chemical equation is one in which the number and type of atoms present in the reactants (the substances on the left of the arrow) equal the number and type of atoms present in the products (the substances on the right of the arrow).
- State symbols like (s), (aq), (l) and (g) are put in brackets after each substance.
- To balance a chemical equation the formulae of the substances must not be changed. This means the small numbers in a formula, for example, the 2 in H2O, may not be changed. Only the number that represents the number of molecules or formula units may change. This is the number in front of the formula.
1.3 Energy transfer in chemical change
- Just as mass and the number of atoms are conserved during a chemical change, so too is the amount of energy of the system.
- During a chemical reaction, energy is needed to break old bonds between atoms, and energy is released when new bonds form between atoms.
- If the energy released is greater than the amount needed to break the old bonds, the extra energy is given out as heat. The container in which the reaction occurs gets hot. This type of reaction is called an exothermic reaction.
- If the energy required to break the bonds is greater than the energy released when the new bonds form, energy must be put into the system. The container in which the reaction happens gets cold. This type of reaction is called an endothermic reaction.
2 Reactions in aqueous solution
2.1 Ions in aqueous solution
- Ions are elements (or groups of elements) that have lost or gained electrons.
- Negative ions are called anions and positive ions are called cations.
- An aqueous solution is a solution in which the solvent (the liquid that dissolves the solid (the solute)) is water.
- Ions in general dissolve easily in water. This process is called dissolution.
- When an ionic solid (a solid made from the bonding of positive and negative ions) is placed in water, dissolution (or the dissolving process) happens in two steps:
the ionic solid breaks up into positive and negative ions
the ions become hydrated. - Dissolution can be shown by means of state symbols. Example: NaCl(s) → Na+(aq) + Cl–(aq)
- In a water molecule, the hydrogen atoms are slightly positively charged, and the oxygen ions are slightly negatively charged. This is called a polar molecule.
- When the ions come into contact with the water, water molecules surround the negative ions. There is an attraction between the negative ion and the hydrogen atoms.
- Positive ions become surrounded because of the attraction between the positive ion and the negative oxygen of the water molecule.
- As a result, the ions cannot come together again, and so they remain in solution.
2.2 Electrolytes and ionisation
- An electrolyte is a solution that can conduct electricity. Ionic solutions are electrolytes.
- To measure the conductivity of an ionic solution, carbon electrodes are used that are attached to either end of a battery. An electrode is defined as a solid object through which electricity enters or leaves a substance.
- Cations flow towards the negative electrode and anions flow towards the positive electrode.
- The more dissolved ions there are in a solution, the greater the current that will flow in it.
- Non-ionic solutions such as sucrose (even though they can dissolve in water) cannot act as an electrolyte and conduct electricity. Solid ionic compounds also cannot conduct electricity. They have to be molten (melted) or in solution.
2.3 Precipitation reactions
- While many ionic compounds are soluble in water, some are not. There are some general rules we can use to predict whether a particular ionic compound will dissolve or not.
Compound Solubility | |
All nitrates | All are soluble. |
Salts containing potassium, sodium or ammonium | All are soluble. |
Chlorides | All are soluble, except silver, lead and mercury chloride. |
Sulphates | All are soluble, except lead sulphate, barium sul- phate and calcium sulphate. |
Carbonates | All are insoluble, except those with potassium sodium or ammonium. |
Silver bromide and silver iodide are insoluble. | |
- If two different ionic solutions are mixed, an ion from the one compound can change places with an ion from the other compound. A chemical reaction occurs. If the water from the solution is removed, two new substances will be present.
Example: BaNO3(aq) + K2SO4(aq) → BaSO4(s) + KNO3(aq)
The NO3– ion has changed places with the SO4– ion. A reaction like this is called an ion-exchange reaction. - If one of the new substances that forms is insoluble, it will form a solid and sink to the bottom of the solution. The solid that forms in such a reaction is called a precipitate.
- Chlorides, bromides and iodides (halides) will all form a precipitate if they are mixed with soluble silver nitrate. Chlorides form a white precipitate. Bromides and iodides both form creamy yellow precipitates, but the precipitate of bromides dissolves in ammonium hydroxide solution.
- Sulphates will form a precipitate if mixed with soluble barium chloride.
Example: MgSO4(aq) + BaCl2(aq) MgCl2(aq) + BaSO4(s) - Carbonates will also form a precipitate with barium chloride. In the case of carbonates, the precipitate that forms will dissolve, producing bubbles, if some nitric acid is added to it.
2.2 Other chemical reaction types
- Precipitation reactions – the driving force is the formation of an insoluble salt. Example: NaCl(aq) + AgNO3(aq) → NaNO3(aq) + AgCl(s)
As you can see from the state symbols, the insoluble salt silver chloride forms during the reaction. - Gas-forming reactions – the driving force is the formation of a gas. Example: Mg(s) + 2 H2O(l) → Mg(OH)2(aq) + H2(g)
The water splits into H+ and OH– ions and the OH– ions join with the magnesium
so that hydrogen is released to form hydrogen gas. - Acid-base reactions – the driving force is the transfer of protons (H+ ions). Example: HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
The H+ ion in the acid reacts with the OH– ion in the base, causing the formation of water. Generally, the product of this reaction is some ionic salt and water. - Redox reactions – the driving force is the transfer of electrons. (You will learn much more about these reactions in a later grade.)
Example: 2Mg(s) + O2(g) → 2MgO(s)
3 Quantitative aspects of chemical change
3.1 Atomic mass and the mole concept
- In chemistry, a ‘mole’ is the unit used to measure the quantity (the number of particles) of a substance.
- One mole of any substance = 6,02 × 1023 particles. This number is known as ‘Avogadro’s number.’
- A mole is also defined as being that quantity of a substance that contains exactly the same number of particles as there are carbon atoms in 12 g of carbon. Therefore, one mole of any element has a mass (molar mass) equal to its relative atomic mass in grams.
- The relative formula mass (of an ionic substance) or the relative molecular mass of a molecule (a covalent substance) is the sum of the atomic masses of all the atoms in its formula. Therefore, the molar mass of a molecular substance is equal to its molecular mass and the molar mass of an ionic substance is equal to its formula mass.
3.2 Molecular and formula masses
3.2.1 Relationship between moles, mass and molar mass
The number of moles = total mass of substances
relative atomic mass of one atom of the substance
OR
n = m / M where n = number of moles; m = the mass of a substance in grams and M is the atomic/formula mass.
3.2.2 Water of crystallisation
- ‘Water of crystallisation’ refers to water molecules that are trapped in some crystals as their crystal lattice forms. Although they are not bonded to the crystal, they add to its mass. Therefore, the water of crystallisation must be added in when the formula mass is calculated.
Example:
Formula mass of CuSO4.5H2O is 4 × (65,5 + 32 + 16) + 5 × (1 × 2 + 16) = 252
3.3 Determining the composition of substances
3.3.1 Percentage composition
- The percentage composition of a compound is the percentage of the total mass of the substance that is made up of each element.
- To calculate the percentage mass of an element in a compound, use the following formula:
% mass = atomic mass + number of atoms of that element × 100
formula mass of the whole compound
[Example: to find the % sodium in Na2CO3
% sodium = 23 × 2 × 100 = 46 × 100 = 43,4%
(23 × 2) + 12 + (16 × 3) 106
3.3.2 Empirical formula
- The empirical formula of a compound is the simplest ratio of all the elements present in that compound. Sometimes the empirical formula and the molecular formula are the same (for example, in H2O), but if the molecular formula can be divided by one common factor (for example, in C4H8), the molecular and empirical formulas are different.
- To find the molecular formula from the empirical formula and the molecular mass:
- Calculate the relative formula mass of the empirical formula.
- Divide this into the molecular mass.
- Take that answer and multiply each subscript in the empirical formula by that number.
Example: The empirical formula is HO and the molecular mass is 34. The relative molecular mass of the empirical formula is (1 + 16). 34 ÷ 17 = 2.
Therefore HO × 2 = H2O2, which is the molecular formula.
3.3.3 Molar volume
- Molar volume of gases refers to the fact that one mole of any gas at the same temperature and pressure has the same volume as one mole of any other gas at that temperature and pressure.
- It has been found that one mole of any gas at standard temperature (which is 273 K or 0 °C) and standard pressure (which is 1,013 × 105 Pa) has a volume of 22,4 dm3. Therefore, the volume of any gas (at STP) = n × 22,4 dm3, where n = the number of moles.
3.3.4 Concentration
- The concentration of a solution is usually given in ‘moles per dm3’ or mol.dm–3.
- Concentration c = n / v where:
c = concentration in mol.dm–3; n = number of moles and v = volume in dm–3. (Note that 1000 cm3 = 1 dm3 = 1000 ml or 1 litre.)
3.4 Basic stoichiometric calculations
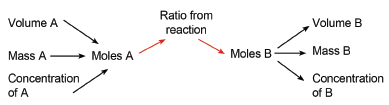
- Using balanced chemical equations and what you have learnt about moles, it is possible to find out how much of a certain product can be made from a certain amount of reactant.
- A balanced reaction gives you the ratio (in moles) in which substances react.
- Example: 2 H2 + O2 → 2 H2O tells us that 2 moles of hydrogen react with 1 mole of oxygen to form 2 moles of water.
- If you are given the mass of reactants and have to find the mass of the products, use the formula (n = mass / M) to convert mass of reactant to moles of reactant.
- Use the ratio given by the reaction to find the number of moles of product formed.
- Convert the number of moles of product to mass of product, again using (n = mass / M).
- If you are given dm3 of a gas at STP, you must first convert this to the number of moles, using the formula volume in dm3 = n × 22,4. (One mole of any gas at STP has a volume of 22,4 dm3.)
- If you are given the concentration of a reactant, convert this to moles using the formula n = c × V.