Matter and Materials - Physical Science Grade 10 Study Guide
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupOverview
Summary
1 Revision of matter (Grade 9)
1.1 Matter and classification
- Matter is made up of particles (atoms or molecules) and it is the properties of the atoms or molecules that determine the characteristics and reactivity of that matter.
- The properties of matter include: strength; density; melting and boiling points; whether the material is brittle, malleable or ductile; whether it is magnetic or not; and its electrical and thermal conductivity.
- Elements and compounds are classified as pure because they contain particles that are all the same. Substances can be classified as pure – elements and compounds
– or mixtures. Pure substances contain particles that are all the same. Elements and compounds can be represented by symbols and formulae. Some of the names, symbols or formulae of a number of common elements and compounds that you should have learnt are the following:- Elements: S for sulphur; C for carbon; P for phosphorus (note the spelling); H for hydrogen; O for oxygen; He for helium; N for nitrogen; F for fluorine; Mg for magnesium; Ca for calcium; Zn for zinc.
- Compounds: H2O for water; H2SO4 for sulphuric acid; HCl for hydrochloric acid; NaCl for sodium chloride (table salt); CaCO3 for calcium carbonate; KNO3 for potassium nitrate; Na3PO4 for sodium phosphate.
- You will notice that the naming of compounds follows these rules:
- The metal or hydrogen is always named first, then the non-metal (potassium iodide: KI; hydrogen chloride: HCl (also called hydrochloric acid)).
- The name of a non-metal ion of an element always ends in –ide (for instance, sodium chloride: NaCl).
- The name of a non-metal complex ion (more than one element in the ion) usually ends in –ate or –ite (for instance, copper sulphate: CuSO4; potassium sulphite: K2SO3). An exception to this is hydroxide (for instance, sodium hydroxide: NaOH).
- Mixtures consist of different substances, and can be homogeneous (have the same ‘look’ throughout) or heterogeneous (you can see the different substances). They can be separated in various ways, for instance filtering, sieving, chromatography, distilling or dissolving one constituent.
- Some of the properties of metals are that they conduct electricity and heat, and they are malleable and ductile.
- Non-metals are non-conductors of electricity and heat. They are gases, liquids or brittle solids, and bond with each other.
- Metalloids are elements on the border between metals and non-metals, and they have both metal and non-metal properties.
2 States of matter and the kinetic molecular theory
- The kinetic molecular theory, together with an understanding of intermolecular forces, explains how matter can exist as solids, liquids or gases. The kinetic molecular theory can also be used to explain diffusion and Brownian motion.
- The kinetic molecular theory says that:
- Matter is made up of particles that are constantly moving.
- Particles have energy that varies according to whether they are in the gas (most energy), liquid or solid (least energy) phase.
- The temperature of a substance is a measure of the average kinetic energy of its particles.
- A change in phase may occur when the energy of the particles is changed by heating or cooling.
- There are spaces between the particles. These are greatest in the gas phase and least in the solid phase.
- Melting (solid → liquid or liquid → solid) points and boiling (liquid → gas or gas → liquid) points are the temperatures at which phase changes happen. They are also specific to pure substances.
- Sublimation is the change from solid to gas without going through the liquid phase, for instance, solid CO2 (dry ice) sublimates to CO2 (gas).
- Brownian motion is the random movement of gas and liquid particles. (‘Random’ means that any one particle can be moving in any possible direction at that instant.)
- Diffusion occurs mostly in gases and liquids. It is the movement of particles of one kind from an area where there are many to an area where there are few. This can happen because of the random movement of all particles and because there are large spaces between particles in the gas and liquid phases.
3 The atom – the basic building block of matter
3.1 The models of the atom
Matter is any substance that has a mass and occupies a volume. A number of models have been developed by different scientists explaining the structure of these atoms. Some of these models are described below:
- Democritus – developed the particle theory of matter. He stated that if you kept dividing something, eventually you would get to a point where it can no longer be divided. He called this indivisible substance ‘atomos’.
- Dalton – said all matter consists of atoms that cannot be made or destroyed. Atoms of the same element are all the same and atoms can be joined together.
- Thomson – developed the ‘currant bun’ model. An atom consists of a solid, positively charged mass in which tiny negatively charged particles are scattered.
- Rutherford – said an atom contains a central, positively charged mass known as the nucleus.
- Bohr – said negatively charged electrons are contained within certain areas in the atom known as ‘energy shells’.
- Schrödinger – developed the ‘wave model’ of the atom in which atoms behave as waves rather than as particles.
3.2 The structure of the atom
The atom consists of a central nucleus containing positively charged protons and neutral neutrons. Together these are known as nucleons.
Surrounding the nucleus is a number of energy shells containing negatively charged electrons.
The number of protons in the nucleus is called the atomic number (Z). In a neutral atom, the number of protons is equal to the number of electrons.
The number of protons and neutrons (added together) in the nucleus is called the mass number (A).
A = Z + N
Mass number Atomic number Number of neutrons
- An atom can either gain or lose electrons in order to form a charged particle, known as an ion. A positive ion is formed when an atom loses electrons, while a negative ion is formed when an atom gains electrons.
- The mass of an atom (atomic mass) is determined by the number of protons and neutrons. Mass of a proton = mass of a neutron = 1 mass unit.
3.3 Isotopes
- Isotopes are atoms of the same element that contain the same number of protons, but different numbers of neutrons. They have the same atomic number, but different mass numbers.
- In the periodic table, two numbers are given with the symbol of each element. These numbers are the atomic number and the average atomic mass.
- In any element, the ratio of the isotopes is constant. The atomic mass is given as the average mass of all the atoms in the sample of the element.
- We can use the percentage composition of the isotopes of an atom to determine the average mass of the element.
- Example: 75% of all naturally occurring chlorine atoms have an atomic mass of 35, while 25% have an atomic mass of 37. We can use these values to calculate the average atomic mass that will appear on the periodic table. We do this by multiplying the percentages of each isotope by their respective atomic masses and then dividing the answer by 100. Atomic mass of chlorine = [(75 × 35) + (25 × 37)] / 100 = 35,5.
3.4 Electron configuration
- Electron configuration refers to the arrangement of electrons in an atom. Electrons occupy orbitals arranged within energy shells (numbered 1, 2, 3 …) around the nucleus. These correspond to the periods in the periodic table.
- Orbitals are regions where electrons spend most of their time. Each orbital can contain a maximum of two electrons.
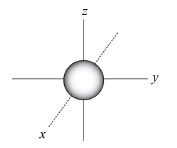
THE S-ORBITAL is spherical (ball-shaped).
EACH ENERGY shell has only one s-orbital.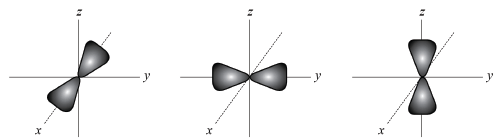
P-ORBITALS ARE shaped like dumb-bells.
THERE ARE no p-orbitals in the first shell.
THE OTHER shells each contain three p-orbitals, named px, py and pz.
EACH ORBITAL can hold two electrons, which means that the three p-orbitals in a shell can hold six electrons in total.
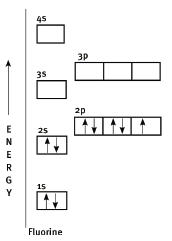
- Scientists use Aufbau diagrams (arrows in boxes) to represent the electron configuration of an atom.
- The shell closest to the nucleus is number 1, the next number is 2, and so on.
Energy level | Types of orbitals | Maximum number of electrons contained |
1 | Only an s-orbital | 2 electrons in the s-orbital |
2 | An s- and 3 p-orbitals | 2 electrons in the s-orbital and 6 electrons in the p-orbitals |
3 | An s- and 3 p-orbitals | 2 electrons in the s-orbital and 6 electrons in the p-orbitals |
Example: An Aufbau diagram for fluorine
- Fluorine contains nine electrons. This means its electron configuration is 1s22s22p5. Each orbital must contain the maximum number of electrons before you can move on to the next orbital.

- As you can see, each electron is represented by an arrow.
- Rules for drawing Aufbau diagrams:
- You must always fill up the lower orbitals before you can move on to the next energy level.
- Pauli’s exclusion principle: ‘Each orbital may contain a maximum of 2 electrons, spinning oppositely’. This is why in each orbital the one arrow faces up, while the other faces down.
- Hund’s rule: ‘Electrons occupy equivalent orbitals (like the p-orbitals in a shell) singly, before pairing takes place’.
4 The periodic table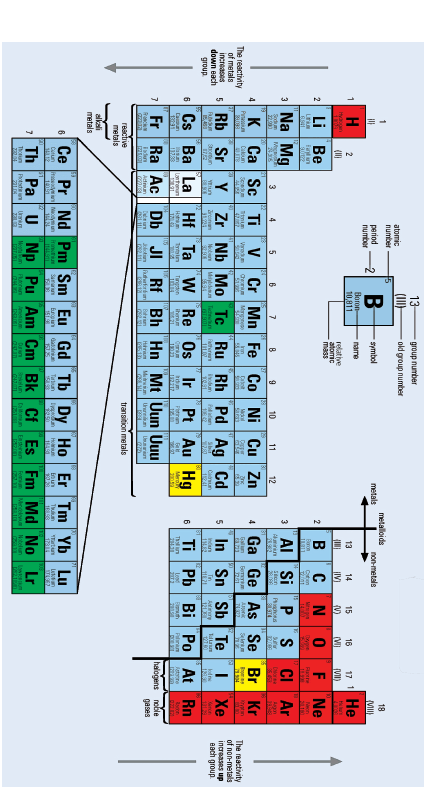
- The elements are arranged in order of increasing atomic number.
- The zig-zag (step) line separates the metals (on the left) from the non-metals (on the right).
- The numbered columns are called groups and are numbered from I to VIII. If the transition metals are included, then the numbers run from 1 to 18.
- The numbered rows are called periods. This number tells you how many energy levels or electron shells there are.
- In some groups the properties of the elements in the group are similar (for instance, the alkali metals of group I, the non-metal halogens of group VII)
- The properties change gradually as you move across a period (for instance, change from metal to non-metal).
- Characteristics that change across the periodic table include the following:
- Atomic radius: This is the distance from the centre of the nucleus to the outer energy shell, and it increases down a group and across a period.
- Ionisation energy: This is the energy needed to remove an electron from an atom of that element in the gas phase, and it increases across a period but decreases down a group.
- Electron affinity: This is the energy change when an electron is added to an atom of an element in the gas phase, and it increases across a period and down a group.
- Electronegativity: This is the ability of an atom to attract the electrons making a bond it is involved in, and it increases across a period but decreases down a group.
5 Chemical bonding
- The electrons found in the outermost energy shells are known as valence electrons and they control how an atom reacts.
- The number of valence electrons equals the group number.
- All atoms try to get a full outer shell of electrons. They can achieve this by gaining electrons from another atom, losing electrons to another atom or sharing electrons with another atom.
- Covalent and ionic bonding are shown using Lewis diagrams.
5.1 Lewis diagrams (dot-cross diagrams)
- Dots or crosses are used to represent the valence electrons of an atom.
- Rules for drawing Lewis diagrams:
- Write the symbol for the element and then draw a cross or dot on top of the symbol.
- Work in a clockwise direction.
- The electrons are only paired once the first four dots or crosses have been drawn.
5.2 Formation of ions
- An ion is a charged particle that forms when a neutral atom either gains or loses electrons.
- Atoms lose or gain electrons in order to obtain a full outer energy shell.
Group | Charge on the ion |
I | +1 |
II | +2 |
III | +3 |
IV | These elements do not form ions. |
V | –3 |
VI | –2 |
VII | –1 |
Elements in group VIII will not form ions, as they already have a full outer shell of electrons.
5.3 Ionic bonding
- Ionic bonding occurs between metals, which form positive ions, and non-metals, which form negative ions. Ionic bonding follows these rules:
- The metal will form a positive ion with a charge equal to the number of electrons it has lost.
- The non-metal ion is written in square brackets. Its charge is equal to the number of electrons gained.
- The square brackets must contain the transferred electrons as well as those of the non-metal ion.

5.4 Covalent bonding
- A covalent bond is formed by overlapping orbitals that share of a pair of electrons. In this way, both atoms obtain a full outer energy shell.
- Water is an example of a covalent compound. Each oxygen atom requires two electrons in order to obtain a full energy shell. This means that two hydrogen atoms are needed. In this case, two single bonds are formed.

- An oxygen molecule contains a double bond, as each oxygen atom shares two electrons. It can be written as O=O.
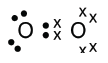
5.5 Metallic bonding
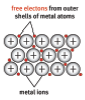
- The valence electrons of metals are loosely held by the nucleus. They move out of position, and become ‘delocalised’ or free electrons.
- The structure is held in place by the strong electrostatic force between the positively charged metal ions and the negatively charged delocalised electrons.
5 Particles that make up substances
6.1 Covalent molecular substances
- Molecules are formed when non-metal atoms are covalently bonded together.
- We can represent molecules by using different circles. Each atom is represented by a circle of a different colour and size.

6.2 Ionic substances
- Ionic substances, also known as salts, are formed when a metal atom transfers electrons to a non-metal atom.
- The electrostatic attraction between the positive and negative ions is what holds the crystal lattice together.
6.3 Covalent network structures
- Covalent network structures are giant molecular compounds formed by a large number of atoms that are covalently bonded together to form a highly regular lattice.
- Diamond and graphite are examples of such covalent network structures.
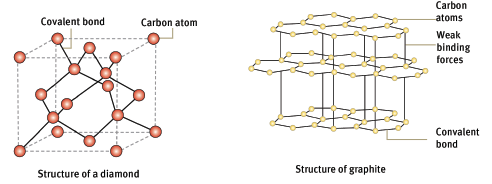
- In diamonds, each carbon atom is attached to four other carbon atoms.
- Graphite consists of layers of carbon atoms. Each carbon atom is attached to three other atoms within the layer. The layers are weakly bonded together and can slide apart easily, which means that graphite is soft. Graphite is used in pencils and as a lubricant in machinery.
6.4 Metallic substances
- A metal substance is one that forms when a group of atoms has a pool of delocalised electrons that surround a lattice of regularly spaced positive ions.
- Most metals are shiny and hard.
6.5 Summary
Bonding: | Ionic: (between metals and non-metals) | Covalent (between non- metals) | Metallic (between metals) | |
Structure: | Giant ionic | Covalent network | Molecular | Giant metallic |
Example: | Sodium chloride
| Diamond
| Iodine
| Zinc
|